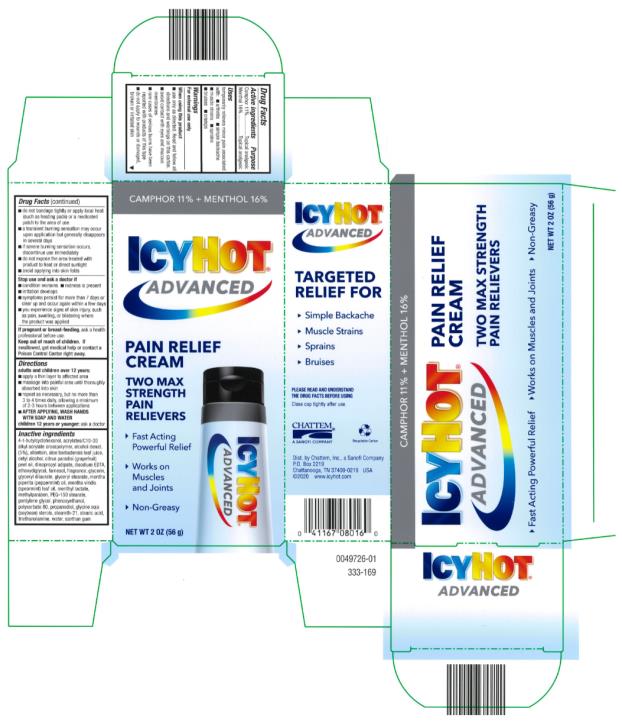
Label: ICY HOT ADVANCED RELIEF- camphor and menthol cream
- NDC Code(s): 41167-0801-0
- Packager: Chattem, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- use only as directed
- do not bandage tightly or use with a heating pad, or medicated patch
- avoid contact with eyes and mucous membranes
- do not apply to wounds or damaged, broken or irritated skin
- a transient burning sensation may occur upon application but generally disappears within a few days
- use only as directed
-
Directions
adults and children over 12 years:
- apply a thin layer to affected area
- message into painful area until thoroughly absorbed into skin
- repeat as necessary, but no more than 3 to 4 times daily, allowing a minimum of 2-3 hours between applications
- AFTER APPLYING, WASH HANDS WITH SOAP AND WATER
Children 12 years and younger: ask a doctor
- apply a thin layer to affected area
-
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, allantoin, aloe barbadensis leaf juice, cetyl alcohol, diisopropyl adipate, disodium EDTA, ethoxydiglycol, fragrance, glycerin, glyceryl stearate, menthyl lactate, methylparaben, PEG-150 sterate, pentylene glycol, 4-t-butylcyclohexanol, phenoxyethanol, polysorbate 80, propanediol, SD alcohol (5% w/w), glycine soja (soybean) sterols, steareth-21, stearic acid, triethanolamine, water, xanthan gum (309-018)
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ICY HOT ADVANCED RELIEF
camphor and menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:41167-0801 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 0.11 g in 1 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 0.16 g in 1 g Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) ALOE VERA LEAF (UNII: ZY81Z83H0X) CETYL ALCOHOL (UNII: 936JST6JCN) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) EDETATE DISODIUM (UNII: 7FLD91C86K) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) METHYLPARABEN (UNII: A2I8C7HI9T) PEG-150 STEARATE (UNII: 7BSG7DF10Q) PENTYLENE GLYCOL (UNII: 50C1307PZG) 4-TERT-BUTYLCYCLOHEXANOL (UNII: K0H1405S9C) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPANEDIOL (UNII: 5965N8W85T) SOY STEROL (UNII: PL360EPO9J) STEARETH-21 (UNII: 53J3F32P58) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) XANTHAN GUM (UNII: TTV12P4NEE) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:41167-0801-0 1 in 1 CARTON 12/01/2012 1 56 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/01/2012 Labeler - Chattem, Inc. (003336013) Establishment Name Address ID/FEI Business Operations CHATTEM, INC. 830410721 analysis(41167-0801) , label(41167-0801) , manufacture(41167-0801) , pack(41167-0801)