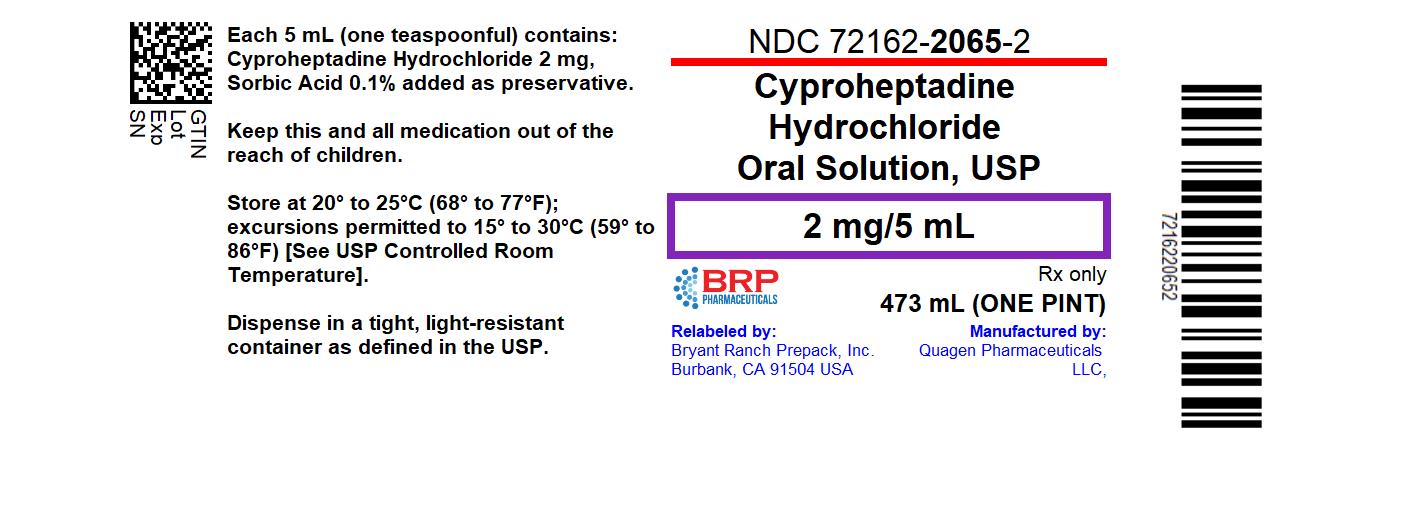
Label: CYPROHEPTADINE HYDROCHLORIDE solution
- NDC Code(s): 72162-2065-2
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 70752-101
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated April 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
Each 5 mL (one teaspoonful) contains: Cyproheptadine Hydrochloride 2 mg
Inactive Ingredients: Citric acid, D&C Yellow #10, peppermint flavor, purified water, propylene glycol, sodium citrate, sorbic acid (0.1% as preservative) and sucrose syrup.Inactive ingredients for Cyproheptadine Oral Solution (Sugar Free): alcohol 5% (v/v), butylated hydroxyanisole, citric acid, D&C Yellow #10, peppermint flavor, propylene glycol, purified water, sodium citrate, sorbic acid (0.1%, as preservative), sucralose.
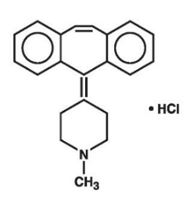
Cyproheptadine HCl is an antihistaminic and antiserotonergic agent. Cyproheptadine hydrochloride is a white to slightly yellowish, crystalline solid, with a molecular weight of 350.89, which is slightly soluble in water, freely soluble in methanol, sparingly soluble in ethanol, soluble in chloroform and practically insoluble in ether. It is the sesquihydrate of 4-(5H-dibenzo[a,d]cyclohepten-5-ylidene)-1-methylpiperidine hydrochloride. The molecular formula of the anhydrous salt is C21H21N•HCl and the structural formula of the anhydrous salt is:
-
CLINICAL PHARMACOLOGY
Cyproheptadine is a serotonin and histamine antagonist with anticholinergic and sedative effects. Antiserotonin and antihistamine drugs appear to compete with serotonin and histamine, respectively, for receptor sites.
Pharmacokinetics and Metabolism: After a single 4 mg oral dose of 14C-labeled cyproheptadine HCI in normal subjects, given as tablets or syrup, 2-20% of the radioactivity was excreted in the stools. Only about 34% of the stool radioactivity was unchanged drug, corresponding to less than 5.7% of the dose. At least 40% of the administered radioactivity was excreted in the urine. No detectable amounts of unchanged drug were present in the urine of patients on chronic 12-20 mg daily doses of cyproheptadine syrup. The principal metabolite found in human urine has been identified as a quaternary ammonium glucuronide conjugate of cyproheptadine. Elimination is diminished in renal insufficiency.
-
INDICATIONS AND USAGE
Perennial and seasonal allergic rhinitis
Vasomotor rhinitis
Allergic conjunctivitis due to inhalant allergens and foods
Mild, uncomplicated allergic skin manifestations of urticaria and angioedema
Amelioration of allergic reactions to blood or plasma
Cold urticaria
DermatographismAs therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
-
CONTRAINDICATIONS
Newborn or Premature Infants: This drug should not be used in newborn or premature infants.
Nursing Mothers: Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Other Conditions:
Hypersensitivity to cyproheptadine and other drugs of similar chemical structure
Monoamine oxidase inhibitor therapy (see Drug Interactions)
Angle-closure glaucoma
Stenosing peptic ulcer
Symptomatic prostatic hypertrophy
Bladder neck obstruction
Pyloroduodenal obstruction
Elderly, debilitated patients -
WARNINGS
Children: Overdosage of antihistamines, particularly in infants and children, may produce hallucinations, central nervous system depression, convulsions and death.
Antihistamines may diminish mental alertness; conversely, particularly in the young child, they may occasionally produce excitation.
CNS Depressants: Antihistamines may have additive effects with alcohol and other CNS depressants, e.g., hypnotics, sedatives, tranquilizers, antianxiety agents.
Activities Requiring Mental Alertness: Patients should be warned about engaging in activities requiring mental alertness and motor coordination, such as driving a car or operating machinery.
Antihistamines are more likely to cause dizziness, sedation and hypotension in elderly patients.
-
PRECAUTIONS
General: Cyproheptadine has an atropine-like action and, therefore, should be used with caution in patients with:
History of bronchial asthma
Increased intraocular pressure
Hyperthyroidism
Cardiovascular disease
Hypertension
Information for Patients: Antihistamines may diminish mental alertness; conversely, particularly in the young child, they may occasionally produce excitation. Patients should be warned about engaging in activities requiring mental alertness and motor coordination, such as driving a car or operating machinery.
Drug Interactions: MAO inhibitors prolong and intensify the anticholinergic effects of antihistamines. Antihistamines may have additive effects with alcohol and other CNS depressants, e.g., hypnotics, sedatives, tranquilizers, antianxiety agents.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term carcinogenic studies have not been done with cyproheptadine. Cyproheptadine had no effect on fertility in a two-litter study in rats or a two-generation study in mice at about 10 times the human dose. Cyproheptadine did not produce chromosome damage in human lymphocytes or fibroblasts in vitro; high doses (10-4M) were cytotoxic. Cyproheptadine did not have any mutagenic effect in the Ames microbial mutagen test; concentrations of above 500 mcg/plate inhibited bacterial growth.
Pregnancy: Pregnancy Category B. Reproduction studies have been performed in rabbits, mice and rats at oral or subcutaneous doses up to 32 times the maximum recommended human oral dose and have revealed no evidence of impaired fertility or harm to the fetus due to cyproheptadine. Cyproheptadine has been shown to be fetotoxic in rats when given by intraperitoneal injection in doses four times the maximum recommended human oral dose. Two studies in pregnant women, however, have not shown that cyproheptadine increases the risk of abnormalities when administered during the first, second and third trimesters of pregnancy. No teratogenic effects were observed in any of the newborns. Nevertheless, because the studies in humans cannot rule out the possibility of harm, cyproheptadine should be used during pregnancy only if clearly needed.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, and because of the potential for serious adverse reactions in nursing infants from cyproheptadine, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother (see CONTRAINDICATIONS).
Pediatric Use: Safety and effectiveness in pediatric patients below the age of two years have not been established. (See CONTRAINDICATIONS, Newborn or Premature Infants, and WARNINGS, Children.)
-
ADVERSE REACTIONS
Adverse reactions which have been reported with the use of antihistamines are as follows:
Central Nervous System: Sedation and sleepiness (often transient), dizziness, disturbed coordination, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, paresthesias, neuritis, convulsions, euphoria, hallucinations, hysteria, faintness.
Integumentary: Allergic manifestation of rash and edema, excessive perspiration, urticaria, photosensitivity.
Special Senses: Acute labyrinthitis, blurred vision, diplopia, vertigo, tinnitus.
Cardiovascular: Hypotension, palpitation, tachycardia, extrasystoles, anaphylactic shock.
Hematologic: Hemolytic anemia, leukopenia, agranulocytosis, thrombocytopenia.
Digestive System: Dryness of mouth, epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation, jaundice.
Genitourinary: Urinary frequency, difficult urination, urinary retention, early menses.
Respiratory: Dryness of nose and throat, thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness.
Miscellaneous: Fatigue, chills, headache, increased appetite/weight gain.
To report SUSPECTED ADVERSE REACTIONS, contact Quagen Pharmaceuticals LLC at 1-888-344-9603 or FDA at 1-800-FDA-1088 or www.FDA.gov/medwatch.
-
OVERDOSAGE
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation especially in children. Also, atropine-like signs and symptoms (dry mouth; fixed, dilated pupils; flushing, etc.) as well as gastrointestinal symptoms may occur.
If vomiting has not occurred spontaneously, the patient should be induced to vomit with syrup of ipecac.
If the patient is unable to vomit, perform gastric lavage followed by activated charcoal. Isotonic or 1/2 isotonic saline is the lavage of choice. Precautions against aspiration must be taken especially in infants and children. When life-threatening CNS signs and symptoms are present, intravenous physostigmine salicylate may be considered. Dosage and frequency of administration are dependent on age, clinical response and recurrence after response. (See package circulars for physostigmine products.)
Saline cathartics, as milk of magnesia, by osmosis draw water into the bowel and, therefore, are valuable for their action in rapid dilution of bowel content.
Stimulants should not be used. Vasopressors may be used to treat hypotension.
The oral LD50 of cyproheptadine is 123 mg/kg, and 295 mg/kg in the mouse and rat, respectively.
-
DOSAGE AND ADMINISTRATION
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
Although intended primarily for administration to children, the syrup is also used for administration to adults who cannot swallow tablets.
Children: The total daily dosage for children may be calculated on the basis of body weight or body area using approximately 0.25 mg/kg/day (0.11 mg/lb/day) or 8 mg per square meter of body surface (8 mg/m2).
Age 2 to 6 years: The usual dose is 2 mg (one teaspoonful) two or three times a day, adjusted as necessary to the size and response of the patient. The dose is not to exceed 12 mg a day.
Age 7 to 14 years: The usual dose is 4 mg (two teaspoonsful) two or three times a day, adjusted as necessary to the size and response of the patient. The dose is not to exceed 16 mg a day.
Adults: The total daily dose for adults should not exceed 0.5 mg/kg/day (0.23 mg/lb/day). The therapeutic range is 4 to 20 mg a day, with the majority of patients requiring 12 to 16 mg a day. An occasional patient may require as much as 32 mg a day for adequate relief. It is suggested that dosage be initiated with 4 mg (two teaspoonsful) three times a day and adjusted according to the size and response of the patient.
-
HOW SUPPLIED
Cyproheptadine Hydrochloride Oral Solution, USP, 2 mg/5 mL in a yellow, peppermint flavor vehicle, is supplied in a pint (473 mL) container (NDC 72162-2065-2).
Store at 20°-25°C (68°-77°F) excursion permitted to 15°-30°C (59°-86°F) [See USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504 - SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CYPROHEPTADINE HYDROCHLORIDE
cyproheptadine hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:72162-2065(NDC:70752-101) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYPROHEPTADINE HYDROCHLORIDE (UNII: NJ82J0F8QC) (CYPROHEPTADINE - UNII:2YHB6175DO) CYPROHEPTADINE HYDROCHLORIDE 2 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SORBIC ACID (UNII: X045WJ989B) SUCROSE (UNII: C151H8M554) PEPPERMINT (UNII: V95R5KMY2B) Product Characteristics Color Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72162-2065-2 473 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 04/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA212423 05/22/2019 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(72162-2065) , RELABEL(72162-2065)