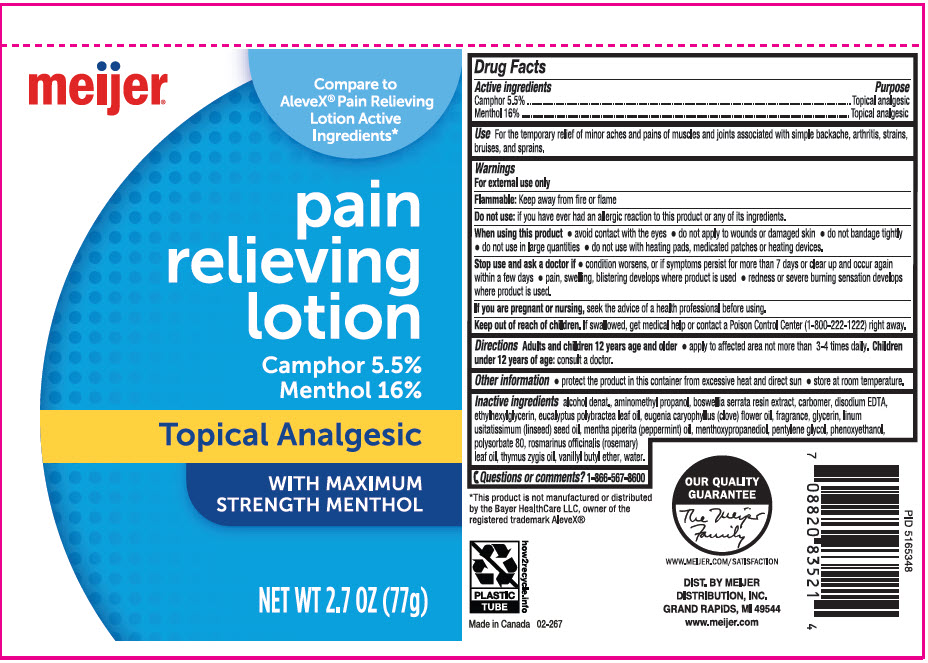
Label: MEIJER PAIN RELIEVING ANALGESIC (camphor- synthetic and menthol, unspecified form lotion
- NDC Code(s): 79481-0051-1
- Packager: Meijer Distribution, INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 22, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Use
-
Warnings
For external use only
Flammable: Keep away from fire or flame
When using this product
- ⬥
- avoid contact with eyes
- ⬥
- do not apply to wounds or damaged skin
- ⬥
- do not bandage tightly
- ⬥
- do not use in large quantities
- ⬥
- do not use with heating pads, medicated patches or heating devices.
- Directions
- Other information
-
Inactive ingredients
alcohol denat., aminomethyl propanol, boswellia serrata resin extract, carbomer, disodium EDTA, ethylhexylglycerin, eucalyptus polybractea leaf oil, Eugenia caryophyllus (clove) flower oil, fragrance, glycerin, linum ultatissimum (linseed) seed oil, mentha piperita (peppermint) oil, menthoxypropanediol, pentylene glycol, phenoxyethanol, polysorbate 80, rosmarinus officinalis (rosemary) leaf oil, thymus zygis oil, vanillyl butyl ether, water.
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 77 g Tube Label
-
INGREDIENTS AND APPEARANCE
MEIJER PAIN RELIEVING ANALGESIC
camphor (synthetic) and menthol, unspecified form lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79481-0051 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 55 mg in 1 g MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 160 mg in 1 g Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) EDETATE DISODIUM (UNII: 7FLD91C86K) Ethylhexylglycerin (UNII: 147D247K3P) Eucalyptus polybractea leaf oil (UNII: J1XGA6WROO) CLOVE OIL (UNII: 578389D6D0) GLYCERIN (UNII: PDC6A3C0OX) LINSEED OIL (UNII: 84XB4DV00W) PEPPERMINT OIL (UNII: AV092KU4JH) 3-((L-MENTHYL)OXY)PROPANE-1,2-DIOL (UNII: KD6TZ2QICH) Pentylene glycol (UNII: 50C1307PZG) Phenoxyethanol (UNII: HIE492ZZ3T) Polysorbate 80 (UNII: 6OZP39ZG8H) ROSEMARY OIL (UNII: 8LGU7VM393) SPANISH THYME OIL (UNII: U14667H96E) Vanillyl butyl ether (UNII: S2ULN37C9R) Water (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79481-0051-1 77 g in 1 TUBE; Type 0: Not a Combination Product 12/05/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 12/05/2022 Labeler - Meijer Distribution, INC (006959555) Registrant - Garcoa, Inc. (036464697) Establishment Name Address ID/FEI Business Operations Sigan Industries INC 255106239 MANUFACTURE(79481-0051)