Label: GUAIFENESIN DM- guaifenesin and dextromethorphan solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 0121-0809-04, 0121-0809-08, 0121-4809-05, 0121-4809-10 - Packager: Pharmaceutical Associates, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 1, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
Stop use and ask a doctor if cough lasts more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- shake well before using
- do not take more than 6 doses in any 24-hour period
age dose adults and children 12 years and over 10 mL (2 teaspoonsful) every 4 hours children 6 to under 12 years of age 5 mL (1 teaspoonful) every 4 hours children 2 to under 6 years of age 2.5 mL (1/2 teaspoonful) every 4 hours children under 2 years consult a doctor
Other information
- each teaspoonful contains: sodium 4 mg
- store at 20° - 25°C (68° - 77°F)
- alcohol/sugar free
- red, cherry flavored solution supplied in the following oral dosage forms: NDC 0121-0809-04 (4 fl oz bottle), NDC 0121-0809-08 (8 fl oz bottle), NDC 0121-4809-05 (unit dose cups of 5 mL, packaged in trays of 10), and NDC 0121-4809-10 (unit dose cups of 10 mL, packaged in trays of 10).
- Inactive ingredients
- Questions or comments?
-
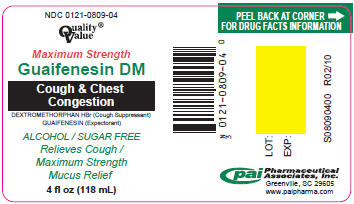
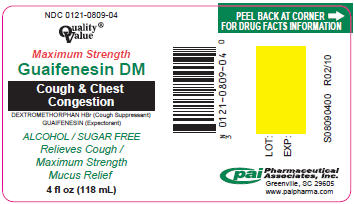
PRINCIPAL DISPLAY PANEL - 118 mL Label
NDC 0121-0809-04
Quality®
ValueMaximum Strength
Guaifenesin DM
Cough & Chest
CongestionDEXTROMETHORPHAN HBr (Cough Suppressant)
GUAIFENESIN (Expectorant)ALCOHOL / SUGAR FREE
Relieves Cough /
Maximum Strength
Mucus Relief4 fl oz (118 mL)
PRINCIPAL DISPLAY PANEL - 5 mL Lid
Delivers 5 mL
NDC 0121-4809-05MAXIMUM STRENGTH
GUAIFENESIN DMGuaifenesin (Expectorant)
Dextromethorphan HBr (Cough Suppressant)200 mg/10 mg per 5 mL
Alcohol Free / Sugar Free
FOR INSTITUTIONAL USE ONLY
PHARMACEUTICAL ASSOCIATES, INC.
GREENVILLE, SC 29605
SEE INSERT
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN DM
guaifenesin and dextromethorphan solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0121-0809 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 5 mL DEXTROMETHORPHAN (UNII: 7355X3ROTS) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN 10 mg in 5 mL Product Characteristics Color RED Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0121-0809-08 237 mL in 1 BOTTLE 2 NDC:0121-0809-04 118 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/17/2010 GUAIFENESIN DM
guaifenesin and dextromethorphan solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0121-4809 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 5 mL DEXTROMETHORPHAN (UNII: 7355X3ROTS) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN 10 mg in 5 mL Product Characteristics Color RED Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0121-4809-05 4 in 1 CASE 1 10 in 1 TRAY 1 5 mL in 1 CUP, UNIT-DOSE 2 NDC:0121-4809-10 4 in 1 CASE 2 10 in 1 TRAY 2 10 mL in 1 CUP, UNIT-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/17/2010 Labeler - Pharmaceutical Associates, Inc. (044940096) Establishment Name Address ID/FEI Business Operations Pharmaceutical Associates, Inc. 044940096 MANUFACTURE