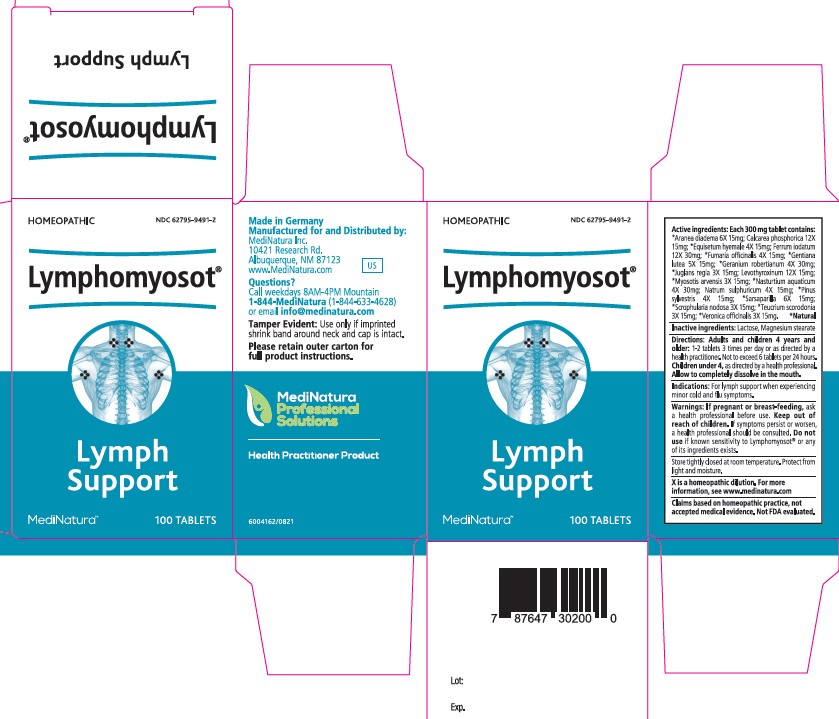
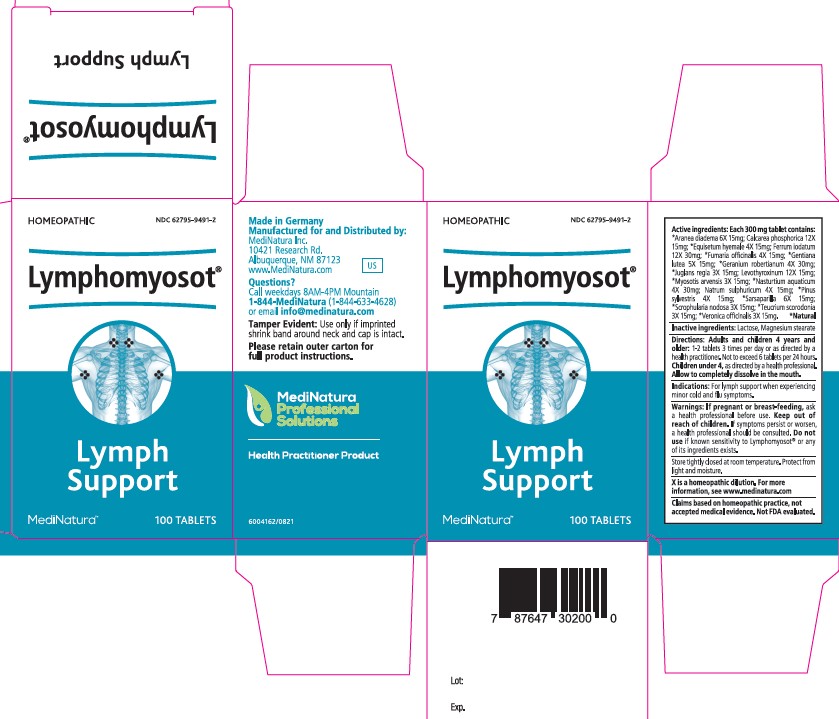
Label: LYMPHOMYOSOT- araneus diadematus, tribasic calcium phosphate,equisetum hyemale whole, ferrous iodide,fumaria officinalis flowering top, gentiana lutea root, juglans regia leaf, levothyroxine sodium anhydrous, myosotis arvensis whole,nasturtium officinale,sodium sulfate, pinus sylvestris leafy twig,smilax ornata root,scrophularia nodosa whole, teucrium scorodonia flowering, veronica officinalis flowering top tablet
- NDC Code(s): 62795-9491-2
- Packager: MediNatura Inc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings:
- KEEP OUT OF REACH OF CHILDREN
- Purpose
-
Active ingredients:
Each 300 mg tablet contains:
*Aranea diadema 6X 15mg; Calcarea phosphorica 12X
15mg; *Equisetum hyemale 4X 15mg; Ferrum iodatum
12X 30mg; *Fumaria officinalis 4X 15mg; *Gentiana
lutea 5X 15mg; *Geranium robertianum 4X 30mg;
*Juglans regia 3X 15mg; Levothyroxinum 12X 15mg;
*Myosotis arvensis 3X 15mg; *Nasturtium aquaticum
4X 30mg; Natrum sulphuricum 4X 15mg; *Pinus
sylvestris 4X 15mg; *Sarsaparilla 6X 15mg;
*Scrophularia nodosa 3X 15mg; *Teucrium scorodonia
3X 15mg; *Veronica officinalis 3X 15mg. *Natural - Inactive ingredients:
- Directions
- Indications:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LYMPHOMYOSOT
araneus diadematus, tribasic calcium phosphate,equisetum hyemale whole, ferrous iodide,fumaria officinalis flowering top, gentiana lutea root, juglans regia leaf, levothyroxine sodium anhydrous, myosotis arvensis whole,nasturtium officinale,sodium sulfate, pinus sylvestris leafy twig,smilax ornata root,scrophularia nodosa whole, teucrium scorodonia flowering, veronica officinalis flowering top tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62795-9491 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARANEUS DIADEMATUS (UNII: 6T6CO7R3Z5) (ARANEUS DIADEMATUS - UNII:6T6CO7R3Z5) ARANEUS DIADEMATUS 6 [hp_X] TRIBASIC CALCIUM PHOSPHATE (UNII: 91D9GV0Z28) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 12 [hp_X] EQUISETUM HYEMALE WHOLE (UNII: 59677RXH25) (EQUISETUM HYEMALE - UNII:59677RXH25) EQUISETUM HYEMALE WHOLE 4 [hp_X] FERROUS IODIDE (UNII: F5452U54PN) (FERROUS IODIDE - UNII:F5452U54PN) FERROUS IODIDE 12 [hp_X] FUMARIA OFFICINALIS FLOWERING TOP (UNII: VH659J61ZL) (FUMARIA OFFICINALIS FLOWERING TOP - UNII:VH659J61ZL) FUMARIA OFFICINALIS FLOWERING TOP 4 [hp_X] GENTIANA LUTEA ROOT (UNII: S72O3284MS) (GENTIANA LUTEA ROOT - UNII:S72O3284MS) GENTIANA LUTEA ROOT 5 [hp_X] GERANIUM ROBERTIANUM (UNII: R5I1HK0UBL) (GERANIUM ROBERTIANUM - UNII:R5I1HK0UBL) GERANIUM ROBERTIANUM 4 [hp_X] JUGLANS REGIA LEAF (UNII: 85HKB87105) (JUGLANS REGIA LEAF - UNII:85HKB87105) JUGLANS REGIA LEAF 3 [hp_X] LEVOTHYROXINE SODIUM ANHYDROUS (UNII: 054I36CPMN) (LEVOTHYROXINE - UNII:Q51BO43MG4) LEVOTHYROXINE SODIUM ANHYDROUS 12 [hp_X] MYOSOTIS ARVENSIS WHOLE (UNII: C73BK97H5J) (MYOSOTIS ARVENSIS - UNII:C73BK97H5J) MYOSOTIS ARVENSIS WHOLE 3 [hp_X] NASTURTIUM OFFICINALE (UNII: YH89GMV676) (NASTURTIUM OFFICINALE - UNII:YH89GMV676) NASTURTIUM OFFICINALE 4 [hp_X] SODIUM SULFATE (UNII: 0YPR65R21J) (SODIUM SULFATE ANHYDROUS - UNII:36KCS0R750) SODIUM SULFATE 4 [hp_X] PINUS SYLVESTRIS LEAFY TWIG (UNII: Q1RGP4UB73) (PINUS SYLVESTRIS LEAFY TWIG - UNII:Q1RGP4UB73) PINUS SYLVESTRIS LEAFY TWIG 4 [hp_X] SMILAX ORNATA ROOT (UNII: 2H1576D5WG) (SARSAPARILLA - UNII:2H1576D5WG) SMILAX ORNATA ROOT 6 [hp_X] SCROPHULARIA NODOSA WHOLE (UNII: 7H443NUB2T) (SCROPHULARIA NODOSA - UNII:7H443NUB2T) SCROPHULARIA NODOSA WHOLE 3 [hp_X] TEUCRIUM SCORODONIA FLOWERING TOP (UNII: LOK3I16O7G) (TEUCRIUM SCORODONIA FLOWERING TOP - UNII:LOK3I16O7G) TEUCRIUM SCORODONIA FLOWERING TOP 3 [hp_X] VERONICA OFFICINALIS FLOWERING TOP (UNII: 9IH82J936J) (VERONICA OFFICINALIS FLOWERING TOP - UNII:9IH82J936J) VERONICA OFFICINALIS FLOWERING TOP 3 [hp_X] Inactive Ingredients Ingredient Name Strength LACTOSE, UNSPECIFIED FORM (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white Score no score Shape ROUND Size 9mm Flavor Imprint Code H Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62795-9491-2 1 in 1 CARTON 02/16/2022 1 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/16/2022 Labeler - MediNatura Inc (079324099) Establishment Name Address ID/FEI Business Operations Biologische Heilmittel Heel 315635359 manufacture(62795-9491)