Label: CYCLOPHOSPHAMIDE injection, solution
- NDC Code(s): 82943-100-03, 82943-101-05
- Packager: Nevakar Injectables Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated July 11, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use CYCLOPHOSPHAMIDE INJECTION safely and effectively. See full prescribing information for CYCLOPHOSPHAMIDE INJECTION.
CYCLOPHOSPHAMIDE INJECTION, for intravenous use
Initial U.S. Approval: 1959INDICATIONS AND USAGE
Cyclophosphamide Injection is an alkylating drug indicated for treatment of adult and pediatric patients with:
- Malignant Diseases: malignant lymphomas: Hodgkin's disease, lymphocytic lymphoma, mixed-cell type lymphoma, histiocytic lymphoma, Burkitt's lymphoma; multiple myeloma, leukemias, mycosis fungoides, neuroblastoma, adenocarcinoma of ovary, retinoblastoma, breast carcinoma. (1)
DOSAGE AND ADMINISTRATION
Malignant Diseases: Adult and Pediatric Patients (2.2)
- Intravenous: Initial course for patients with no hematologic deficiency: 40 mg/kg to 50 mg/kg in divided doses over 2 to 5 days. Other regimens include 10 mg/ kg to 15 mg/ kg given every 7 to 10 days or 3 mg/ kg to 5 mg/ kg twice weekly.
- See full prescribing information for instructions on preparation, handling, and administration. (2.3)
DOSAGE FORMS AND STRENGTHS
Injection: 500 mg/2.5 mL (200 mg/mL) and 1,000 mg/5 mL (200 mg/mL) in a multiple-dose vial (3)
WARNINGS AND PRECAUTIONS
- Myelosuppression, Immunosuppression, Bone Marrow Failure and Infections - Severe immunosuppression may lead to serious and sometimes fatal infections. Close hematological monitoring is required. (5.1)
- Urinary Tract and Renal Toxicity - Hemorrhagic cystitis, pyelitis, ureteritis, and hematuria can occur. Exclude or correct any urinary tract obstructions prior to treatment. (5.2)
- Cardiotoxicity - Myocarditis, myopericarditis, pericardial effusion, arrhythmias and congestive heart failure, which may be fatal, have been reported. Monitor patients, especially those with risk factors for cardio toxicity or pre-existing cardiac disease. (5.3)
- Pulmonary Toxicity - Pneumonitis, pulmonary fibrosis and pulmonary veno-occlusive disease leading to respiratory failure may occur. Monitor patients for signs and symptoms of pulmonary toxicity. (5.4)
- Secondary Malignancies - Have been reported in patients treated with cyclophosphamide-containing regimens. (5.5)
- Veno-occlusive Liver Disease - Fatal outcome can occur. (5.6)
- Alcohol Content - The alcohol content in a dose of Cyclophosphamide Injection may affect the central nervous system. This may include impairment of a patient’s ability to drive or use machines immediately after infusion. (5.7)
- Embryo-Fetal Toxicity - Can cause fetal harm. Advise patients of reproductive potential of the potential risk to a fetus and to use effective contraception. (5.8, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions reported are neutropenia, febrile neutropenia, fever, alopecia, nausea, vomiting, and diarrhea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Nevakar Injectables at 908-367-7400 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1. Important Dosing Information
2.2. Recommended Dosage for Malignant Diseases
2.3. Preparation, Handling and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1. Myelosuppression, Immunosuppression, Bone Marrow Failure and Infections
5.2. Urinary Tract and Renal Toxicity
5.3. Cardiotoxicity
5.4. Pulmonary Toxicity
5.5. Secondary Malignancies
5.6. Veno-occlusive Liver Disease
5.7. Alcohol Content
5.8. Embryo-Fetal Toxicity
5.9. Infertility
5.10. Impairment of Wound Healing
5.11. Hyponatremia
6 ADVERSE REACTIONS
6.1. Clinical Trials and Postmarketing Experience
7 DRUG INTERACTIONS
7.1. Effect of Other Drugs on Cyclophosphamide Exposure
7.2. Drugs that Can Potentiate Cyclophosphamide Toxicities
7.3. Effect of Cyclophosphamide With Other Drugs
8 USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
8.2. Lactation
8.3. Females and Males of Reproductive Potential
8.4. Pediatric Use
8.5. Geriatric Use
8.6. Renal Impairment
8.7. Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
12.2. Pharmacodynamics
12.3. Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Malignant Diseases
Cyclophosphamide Injection is indicated for the treatment of adult and pediatric patients with:
- malignant lymphomas (Stages III and IV of the Ann Arbor staging system), Hodgkin's disease, lymphocytic lymphoma (nodular or diffuse), mixed-cell type lymphoma, histiocytic lymphoma, Burkitt's lymphoma
- multiple myeloma
- leukemias: chronic lymphocytic leukemia, chronic granulocytic leukemia (it is usually ineffective in acute blastic crisis), acute myelogenous and monocytic leukemia, acute lymphoblastic (stem-cell) leukemia (cyclophosphamide given during remission is effective in prolonging its duration)
- mycosis fungoides (advanced disease)
- neuroblastoma (disseminated disease)
- adenocarcinoma of the ovary
- retinoblastoma
- carcinoma of the breast
Cyclophosphamide, although effective alone in susceptible malignancies, is more frequently used concurrently or sequentially with other antineoplastic drugs.
-
2 DOSAGE AND ADMINISTRATION
During or immediately after the administration, adequate amounts of fluid should be ingested or infused to force diuresis in order to reduce the risk of urinary tract toxicity. Therefore, cyclophosphamide should be administered in the morning.
2.1. Important Dosing Information
During or immediately after the administration, adequate amounts of fluid should be ingested or infused to force diuresis in order to reduce the risk of urinary tract toxicity. Therefore, Cyclophosphamide Injection should be administered in the morning.
2.2. Recommended Dosage for Malignant Diseases
Adults and Pediatric Patients
Intravenous
When used as the only oncolytic drug therapy, the initial course of Cyclophosphamide Injection for patients with no hematologic deficiency usually consists of 40 mg/kg to 50 mg/kg given intravenously in divided doses over a period of 2 to 5 days. Other intravenous regimens include 10 mg/kg to 15 mg/kg given every 7 to 10 days or 3 mg/kg to 5 mg/kg twice weekly.
Dosages may also be adjusted based on antitumor activity and/or leukopenia. The total leukocyte count may be used to manage dosage.
When Cyclophosphamide Injection is included in combined cytotoxic regimens, it may be necessary to reduce the dose of Cyclophosphamide Injection as well as that of the other drugs.
2.3. Preparation, Handling and Administration
Cyclophosphamide Injection is a hazardous drug.1 Follow applicable special handling and disposal procedures. Caution should be exercised when handling and preparing Cyclophosphamide Injection. To minimize the risk of dermal exposure, always wear gloves when handling vials containing Cyclophosphamide Injection.
Cyclophosphamide Injection
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Do not use Cyclophosphamide Injection vials if there are signs of particulate matter.
Cyclophosphamide Injection does not contain any antimicrobial preservative and thus care must be taken to assure the sterility of prepared solutions. Use aseptic technique.
For Direct Intravenous Injection
Aseptically withdraw the prescribed dose from the vial. Dilute the prescribed dose of Cyclophosphamide Injection to a concentration of 20 mg per mL by using any of the following diluents:
- 0.9% Sodium Chloride Injection, USP
- 0.45% Sodium Chloride Injection, USP
- 5% Dextrose Injection, USP
- 5% Dextrose and 0.9% Sodium Chloride Injection, USP
Do not use Sterile Water for Injection, USP because it results in a hypotonic solution and should not be injected directly.
For Intravenous Infusion
Aseptically withdraw the prescribed dose from the vial. Dilute the prescribed dose of Cyclophosphamide Injection to a concentration of 2 mg per mL by using any of the following diluents:
- 0.9% Sodium Chloride Injection, USP
- 0.45% Sodium Chloride Injection, USP
- 5% Dextrose Injection, USP
- 5% Dextrose and 0.9% Sodium Chloride Injection, USP
To reduce the likelihood of adverse reactions that appear to be administration rate-dependent (e.g., facial swelling, headache, nasal congestion, scalp burning), Cyclophosphamide Injection should be injected or infused very slowly. Duration of the infusion also should be appropriate for the volume and type of carrier fluid to be infused.
Storage of Diluted Cyclophosphamide Injection Solution:
If not used immediately, for microbiological integrity, cyclophosphamide solutions should be stored as described in Table 1:
Table 1: Storage of Cyclophosphamide Injection Solutions
Diluent
Storage
Room Temperature
Refrigerated
Diluted solutions (20 mg/mL)
0.9% Sodium Chloride Injection, USP
up to 24 hrs
up to 6 days
0.45% Sodium Chloride Injection, USP
up to 24 hrs
up to 6 days
5% Dextrose Injection, USP
up to 24 hrs
up to 6 days
5% Dextrose and 0.9% Sodium Chloride Injection, USP
up to 24 hrs
up to 6 days
Diluted Solutions (2 mg/mL)
0.9% Sodium Chloride Injection, USP
up to 24 hrs
up to 6 days
0.45% Sodium Chloride Injection, USP
up to 24 hrs
up to 6 days
5% Dextrose Injection, USP
up to 24 hrs
up to 6 days
5% Dextrose and 0.9% Sodium Chloride Injection, USP
up to 24 hrs
up to 6 days
Storage of Undiluted Cyclophosphamide Injection Solution:
After first use, store partially used multiple-dose vial in the original carton at 2°C to 8°C (36ºF to 46°F) for up to 28 days. Discard unused portion after 28 days.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Hypersensitivity
Cyclophosphamide is contraindicated in patients who have a history of severe hypersensitivity reactions to it, any of its metabolites, or to other components of the product. Anaphylactic reactions including death have been reported with cyclophosphamide. Possible cross-sensitivity with other alkylating agents can occur.
Urinary Outflow Obstruction
Cyclophosphamide is contraindicated in patients with urinary outflow obstruction [see Warnings and Precautions (5.2)].
-
5 WARNINGS AND PRECAUTIONS
5.1. Myelosuppression, Immunosuppression, Bone Marrow Failure and Infections
Cyclophosphamide can cause myelosuppression (leukopenia, neutropenia, thrombocytopenia and anemia), bone marrow failure, and severe immunosuppression which may lead to serious and sometimes fatal infections, including sepsis and septic shock. Latent infections can be reactivated [see Adverse Reactions (6.1)].
Antimicrobial prophylaxis may be indicated in certain cases of neutropenia at the discretion of the managing physician. In case of neutropenic fever, antibiotic therapy is indicated. Antimycotics and/or antivirals may also be indicated.
Monitoring of complete blood counts is essential during cyclophosphamide treatment so that the dose can be adjusted, if needed. Cyclophosphamide Injection should not be administered to patients with neutrophils ≤1,500/mm3 and platelets < 50,000/mm3. Cyclophosphamide Injection treatment may not be indicated, or should be interrupted, or the dose reduced, in patients who have or who develop a serious infection. G-CSF may be administered to reduce the risks of neutropenia complications associated with cyclophosphamide use. Primary and secondary prophylaxis with G-CSF should be considered in all patients considered to be at increased risk for neutropenia complications. The nadirs of the reduction in leukocyte count and thrombocyte count are usually reached in weeks 1 and 2 of treatment. Peripheral blood cell counts are expected to normalize after approximately 20 days. Bone marrow failure has been reported. Severe myelosuppression may be expected particularly in patients pretreated with and/or receiving concomitant chemotherapy and/or radiation therapy.
5.2. Urinary Tract and Renal Toxicity
Hemorrhagic cystitis, pyelitis, ureteritis, and hematuria have been reported with cyclophosphamide. Medical and/or surgical supportive treatment may be required to treat protracted cases of severe hemorrhagic cystitis. Discontinue cyclophosphamide therapy in case of severe hemorrhagic cystitis. Urotoxicity (bladder ulceration, necrosis, fibrosis, contracture and secondary cancer) may require interruption of cyclophosphamide treatment or cystectomy. Urotoxicity can be fatal. Urotoxicity can occur with short-term or long-term use of cyclophosphamide.
Before starting treatment, exclude or correct any urinary tract obstructions [see Contraindications (4)]. Urinary sediment should be checked regularly for the presence of erythrocytes and other signs of urotoxicity and/or nephrotoxicity. Cyclophosphamide Injection should be used with caution, if at all, in patients with active urinary tract infections. Aggressive hydration with forced diuresis and frequent bladder emptying can reduce the frequency and severity of bladder toxicity. Mesna has been used to prevent severe bladder toxicity.5.3. Cardiotoxicity
Myocarditis, myopericarditis, pericardial effusion including cardiac tamponade, and congestive heart failure, which may be fatal, have been reported with cyclophosphamide therapy.
Supraventricular arrhythmias (including atrial fibrillation and flutter) and ventricular arrhythmias (including severe QT prolongation associated with ventricular tachyarrhythmia) have been reported after treatment with regimens that included cyclophosphamide.
The risk of cardiotoxicity may be increased with high doses of cyclophosphamide, in patients with advanced age, and in patients with previous radiation treatment to the cardiac region and/or previous or concomitant treatment with other cardiotoxic agents.
Particular caution is necessary in patients with risk factors for cardiotoxicity and in patients with pre- existing cardiac disease.
Monitor patients with risk factors for cardiotoxicity and with pre-existing cardiac disease.
5.4. Pulmonary Toxicity
Pneumonitis, pulmonary fibrosis, pulmonary veno-occlusive disease and other forms of pulmonary toxicity leading to respiratory failure have been reported during and following treatment with cyclophosphamide. Late onset pneumonitis (greater than 6 months after start of cyclophosphamide) appears to be associated with increased mortality. Pneumonitis may develop years after treatment with cyclophosphamide.
Monitor patients for signs and symptoms of pulmonary toxicity.
5.5. Secondary Malignancies
Cyclophosphamide is genotoxic [see Nonclinical Toxicology (13.1)]. Secondary malignancies (urinary tract cancer, myelodysplasia, acute leukemias, lymphomas, thyroid cancer, and sarcomas) have been reported in patients treated with cyclophosphamide-containing regimens. The risk of bladder cancer may be reduced by prevention of hemorrhagic cystitis.
5.6. Veno-occlusive Liver Disease
Veno-occlusive liver disease (VOD) including fatal outcome has been reported in patients receiving cyclophosphamide-containing regimens. A cytoreductive regimen in preparation for bone marrow transplantation that consists of cyclophosphamide in combination with whole-body irradiation, busulfan, or other agents has been identified as a major risk factor. VOD has also been reported to develop gradually in patients receiving long-term low-dose immunosuppressive doses of cyclophosphamide. Other risk factors predisposing to the development of VOD include preexisting disturbances of hepatic function, previous radiation therapy of the abdomen, and a low performance status.
5.7. Alcohol Content
The alcohol content in a dose of Cyclophosphamide Injection may affect the central nervous system and should be taken into account for patients in whom alcohol intake should be avoided or minimized. Consideration should be given to the alcohol content in Cyclophosphamide Injection on the ability to drive or use machines immediately after the infusion.
Each administration of Cyclophosphamide Injection at 50 mg per kg delivers 0.166 g/kg of ethanol over 2 to 5 days. For a 75 kg patient this would deliver 12.45 grams of ethanol over 2 to 5 days [see Description (11)]. Other cyclophosphamide products may have a different amount of alcohol or no alcohol.
5.8. Embryo-Fetal Toxicity
Based on its mechanism of action and published reports of effects in pregnant patients or animals, Cyclophosphamide Injection can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1),Clinical Pharmacology (12.1) and Nonclinical Toxicology (13.1)]. Exposure to cyclophosphamide during pregnancy may cause birth defects, miscarriage, fetal growth retardation, and fetotoxic effects in the newborn. Cyclophosphamide is teratogenic and embryo-fetal toxic in mice, rats, rabbits and monkeys.
Advise pregnant women and females of reproductive potential of the potential risk to a fetus [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with Cyclophosphamide Injection and for up to 1 year after completion of therapy. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with Cyclophosphamide Injection and for 4 months after completion of therapy [see Use in Specific Populations (8.1, 8.3)].
5.9. Infertility
Male and female reproductive function and fertility may be impaired in patients being treated with Cyclophosphamide Injection. Cyclophosphamide interferes with oogenesis and spermatogenesis. It may cause sterility in both sexes. Development of sterility appears to depend on the dose of cyclophosphamide, duration of therapy, and the state of gonadal function at the time of treatment. Cyclophosphamide-induced sterility may be irreversible in some patients. Advise patients on the potential risks for infertility [see Use in Specific Populations (8.3 and 8.4)].
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling.
- Hypersensitivity [see Contraindications (4)]
- Myelosuppression, Immunosuppression, Bone Marrow Failure, and Infections [see Warnings and Precautions (5.1)]
- Urinary Tract and Renal Toxicity [see Warnings and Precautions (5.2)]
- Cardiotoxicity [see Warnings and Precautions (5.3)]
- Pulmonary Toxicity [see Warnings and Precautions (5.4)]
- Secondary Malignancies [see Warnings and Precautions (5.5)]
- Veno-occlusive Liver Disease [see Warnings and Precautions (5.6)]
- Alcohol Content [see Warnings and Precautions (5.7)]
- Infertility [see Warnings and Precautions (5.9) and Use in Specific Populations (8.3 and 8.4)]
- Impaired Wound Healing [see Warnings and Precautions (5.10)]
- Hyponatremia [see Warnings and Precautions (5.11)]
6.1. Clinical Trials and Postmarketing Experience
The following adverse reactions associated with the use of cyclophosphamide were identified in clinical studies or post-marketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The most common adverse reactions were neutropenia, febrile neutropenia, fever, alopecia, nausea, vomiting, and diarrhea.
Cardiac: cardiac arrest, ventricular fibrillation, ventricular tachycardia, cardiogenic shock, pericardial effusion (progressing to cardiac tamponade), myocardial hemorrhage, myocardial infarction, cardiac failure (including fatal outcomes), cardiomyopathy, myocarditis, pericarditis, carditis, atrial fibrillation, supraventricular arrhythmia, ventricular arrhythmia, bradycardia, tachycardia, palpitations, QT prolongation.
Congenital, Familial and Genetic: intra-uterine death, fetal malformation, fetal growth retardation, fetal toxicity (including myelosuppression, gastroenteritis).
Ear and Labyrinth: deafness, hearing impaired, tinnitus.
Endocrine: water intoxication.
Eye: visual impairment, conjunctivitis, lacrimation.
Gastrointestinal: gastrointestinal hemorrhage, acute pancreatitis, colitis, enteritis, cecitis, stomatitis, constipation, parotid gland inflammation, nausea, vomiting, diarrhea.
General Disorders and Administrative Site Conditions: multiorgan failure, general physical deterioration, influenza-like illness, injection/infusion site reactions (thrombosis, necrosis, phlebitis, inflammation, pain, swelling, erythema), pyrexia, edema, chest pain, mucosal inflammation, asthenia, pain, chills, fatigue, malaise, headache, febrile neutropenia.
Hematologic: myelosuppression, bone marrow failure, disseminated intravascular coagulation and hemolytic uremic syndrome (with thrombotic microangiopathy).
Hepatic: veno-occlusive liver disease, cholestatic hepatitis, cytolytic hepatitis, hepatitis, cholestasis; hepatotoxicity with hepatic failure, hepatic encephalopathy, ascites, hepatomegaly, blood bilirubin increased, hepatic function abnormal, hepatic enzymes increased.
Immune: immunosuppression, anaphylactic shock and hypersensitivity reaction.
Infections: The following manifestations have been associated with myelosuppression and immunosuppression caused by cyclophosphamide: increased risk for and severity of pneumonias (including fatal outcomes), other bacterial, fungal, viral, protozoal and, parasitic infections; reactivation of latent infections, (including viral hepatitis, tuberculosis), Pneumocystis jiroveci, herpes zoster, Strongyloides, sepsis and septic shock.
Investigations: blood lactate dehydrogenase increased, C-reactive protein increased.
Metabolism and Nutrition: hyponatremia, fluid retention, blood glucose increased, blood glucose decreased.
Musculoskeletal and Connective Tissue: rhabdomyolysis, scleroderma, muscle spasms, myalgia, arthralgia.
Neoplasms: acute leukemia, myelodysplastic syndrome, lymphoma, sarcomas, renal cell carcinoma, renal pelvis cancer, bladder cancer, ureteric cancer, thyroid cancer.
Nervous System: encephalopathy, convulsion, dizziness, neurotoxicity has been reported and manifested as reversible posterior leukoencephalopathy syndrome, myelopathy, peripheral neuropathy, polyneuropathy, neuralgia, dysesthesia, hypoesthesia, paresthesia, tremor, dysgeusia, hypogeusia, parosmia.
Pregnancy: premature labor.
Psychiatric: confusional state.
Renal and Urinary: renal failure, renal tubular disorder, renal impairment, nephropathy toxic, hemorrhagic cystitis, bladder necrosis, cystitis ulcerative, bladder contracture, hematuria, nephrogenic diabetes insipidus, atypical urinary bladder epithelial cells.
Reproductive System: infertility, ovarian failure, ovarian disorder, amenorrhea, oligomenorrhea, testicular atrophy, azoospermia, oligospermia.
Respiratory: pulmonary veno-occlusive disease, acute respiratory distress syndrome, interstitial lung disease as manifested by respiratory failure (including fatal outcomes), obliterative bronchiolitis, organizing pneumonia, alveolitis allergic, pneumonitis, pulmonary hemorrhage; respiratory distress, pulmonary hypertension, pulmonary edema, pleural effusion, bronchospasm, dyspnea, hypoxia, cough, nasal congestion, nasal discomfort, oropharyngeal pain, rhinorrhea.
Skin and Subcutaneous Tissue: toxic epidermal necrolysis, Stevens-Johnson syndrome, erythema multiforme, palmar-plantar erythrodysesthesia syndrome, radiation recall dermatitis, toxic skin eruption, urticaria, dermatitis, blister, pruritus, erythema, nail disorder, facial swelling, hyperhidrosis, alopecia.
Tumorlysis syndrome: like other cytotoxic drugs, cyclophosphamide may induce tumor-lysis syndrome and hyperuricemia in patients with rapidly growing tumors.
Vascular: pulmonary embolism, venous thrombosis, vasculitis, peripheral ischemia, hypertension, hypotension, flushing, hot flush.
-
7 DRUG INTERACTIONS
7.1. Effect of Other Drugs on Cyclophosphamide Exposure
Protease Inhibitors
Cyclophosphamide is a pro-drug that is activated by cytochrome P450s [see Clinical Pharmacology (12.3)].Concomitant use of protease inhibitors may increase the concentration of cytotoxic metabolites. Use of protease inhibitor-based regimens was found to be associated with a higher Incidence of infections and neutropenia in patients receiving cyclophosphamide, doxorubicin, and etoposide (CDE) than use of a Non-Nucleoside Reverse Transcriptase Inhibitor-based regimen.
7.2. Drugs that Can Potentiate Cyclophosphamide Toxicities
Combined or sequential use of Cyclophosphamide Injection and other drugs or agents with similar toxicities to Cyclophosphamide Injection and can potentiate these effects and are listed in Table 2.
Table 2: Drugs that Can Potentiate Cyclophosphamide Toxicities
Toxicity
Drug or other treatment
Increased hematotoxicity and/or immunosuppression
- ACE inhibitors: ACE inhibitors can cause leukopenia.
- Natalizumab
- Paclitaxel: Increased hematotoxicity has been reported when cyclophosphamide was administered after paclitaxel infusion.
- Thiazide diuretics
- Zidovudine
Increased cardiotoxicity
- Anthracyclines
- Cytarabine
- Pentostatin
- Radiation therapy of the cardiac region
- Trastuzumab
Increased pulmonary toxicity
- Amiodarone
- G-CSF, GM-CSF (granulocyte colony-stimulating factor, granulocyte macrophage colony-stimulating factor)
Increased nephrotoxicity
- Amphotericin B
- Indomethacin: Acute water intoxication has been reported with concomitant use of indomethacin
Increase in other toxicities:
- Azathioprine: Increased risk of hepatotoxicity (liver necrosis)
- Busulfan: Increased incidence of hepatic veno-occlusive disease and mucositis has been reported.
- Protease inhibitors: Increased incidence of mucositis
Increased risk of hemorrhagic cystitis
- Radiation treatment: Increased risk of hemorrhagic cystitis may result from a combined effect of cyclophosphamide and past or concomitant radiation treatment.
7.3. Effect of Cyclophosphamide With Other Drugs
Etanercept
A higher incidence of non-cutaneous malignant solid tumors in patients with Wegener’s granulomatosis occurred with the addition of etanercept to cyclophosphamide treatment.
Metronidazole
Acute encephalopathy has been reported in a patient receiving cyclophosphamide and metronidazole. In an animal study, the combination of cyclophosphamide with metronidazole was associated with increased cyclophosphamide toxicity.
Tamoxifen
Concomitant use of tamoxifen and chemotherapy may increase the risk of thromboembolic complications.
Coumarins
Both increased and decreased warfarin effect have been reported in patients receiving warfarin and cyclophosphamide.
Cyclosporine
Lower serum concentrations of cyclosporine have been observed in patients receiving a combination of cyclophosphamide and cyclosporine than in patients receiving only cyclosporine. This interaction may result in an increased incidence of graft-versus-host disease.
Depolarizing muscle relaxants
If a patient has been treated with cyclophosphamide within 10 days of general anesthesia, alert the anesthesiologist.
Cyclophosphamide treatment causes a marked and persistent inhibition of cholinesterase activity. Prolonged apnea may occur with concurrent depolarizing muscle relaxants (e.g., succinylcholine).
-
8 USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Risk Summary
Based on its mechanism of action and published reports of effects in pregnant patients or animals, Cyclophosphamide Injection can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1) and Nonclinical Toxicology (13.1)]. Exposure to cyclophosphamide during pregnancy may cause fetal malformations, miscarriage, fetal growth retardation, and toxic effects in the newborn [see Data]. Cyclophosphamide is teratogenic and embryo-fetal toxic in mice, rats, rabbits and monkeys [see Data]. Advise pregnant women and females of reproductive potential of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects is 2% - 4% and of miscarriage is 15% - 20% of clinically recognized pregnancies.
Data
Human Data
Malformations of the skeleton, palate, limbs and eyes as well as miscarriage have been reported after exposure to cyclophosphamide in the first trimester. Fetal growth retardation and toxic effects manifesting in the newborn, including leukopenia, anemia, pancytopenia, severe bone marrow hypoplasia, and gastroenteritis have been reported after exposure to cyclophosphamide.
Animal Data
Administration of cyclophosphamide to pregnant mice, rats, rabbits and monkeys during the period of organogenesis at doses at or below the dose in patients based on body surface area resulted in various malformations, which included neural tube defects, limb and digit defects and other skeletal anomalies, cleft lip and palate, and reduced skeletal ossification.
8.2. Lactation
Risk Summary
Cyclophosphamide is present in breast milk. Neutropenia, thrombocytopenia, low hemoglobin, and diarrhea have been reported in infants breast fed by women treated with cyclophosphamide. Because of the potential for serious adverse reactions in a breastfed child, advise lactating women not to breastfeed during treatment with Cyclophosphamide Injection and for 1 week after the last dose.
8.3. Females and Males of Reproductive Potential
Cyclophosphamide Injection can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to the initiation of Cyclophosphamide Injection [see Use in Specific Populations (8.1)].
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with Cyclophosphamide Injection and for up to 1 year after completion of therapy [see Use in Specific Populations (8.1)].
Males
Based on findings in genetic toxicity and animal reproduction studies, advise male patients with female partners of reproductive potential to use effective contraception during treatment with Cyclophosphamide Injection and for 4 months after completion of therapy [see Use in Specific Populations (8.1) and Nonclinical Toxicology (13.1)].
Infertility
Females
Amenorrhea, transient or permanent, associated with decreased estrogen and increased gonadotropin secretion develops in a proportion of women treated with cyclophosphamide. Affected patients generally resume regular menses within a few months after cessation of therapy. The risk of premature menopause with cyclophosphamide increases with age. Oligomenorrhea has also been reported in association with cyclophosphamide treatment.
Animal data suggest an increased risk of failed pregnancy and malformations may persist after discontinuation of cyclophosphamide as long as oocytes/follicles exist that were exposed to cyclophosphamide during any of their maturation phases. The exact duration of follicular development in humans is not known but may be longer than 12 months [see Nonclinical Toxicology (13.1)].
Males
Men treated with cyclophosphamide may develop oligospermia or azoospermia which are normally associated with increased gonadotropin but normal testosterone secretion.
8.4. Pediatric Use
The safety and effectiveness of Cyclophosphamide Injection have been established in pediatric patients and information on this use is discussed throughout the labeling.
The alcohol content of Cyclophosphamide Injection should be taken into account when given to pediatric patients [see Warnings and Precautions (5.7)].
Pre-pubescent girls treated with cyclophosphamide generally develop secondary sexual characteristics normally and have regular menses. Ovarian fibrosis with apparently complete loss of germ cells after prolonged cyclophosphamide treatment in late pre-pubescence has been reported. Girls treated with cyclophosphamide who have retained ovarian function after completing treatment are at increased risk of developing premature menopause.
Pre-pubescent boys treated with cyclophosphamide develop secondary sexual characteristics normally, but may have oligospermia or azoospermia and increased gonadotropin secretion. Some degree of testicular atrophy may occur. Cyclophosphamide-induced azoospermia is reversible in some patients, though the reversibility may not occur for several years after cessation of therapy.
8.5. Geriatric Use
There is insufficient data from clinical studies of cyclophosphamide available for patients 65 years of age and older to determine whether they respond differently than younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac functioning, and of concomitant disease or other drug therapy.
8.6. Renal Impairment
In patients with severe renal impairment, decreased renal excretion may result in increased plasma levels of cyclophosphamide and its metabolites. This may result in increased toxicity [see Clinical Pharmacology (12.3)]. Monitor patients with severe renal impairment (CLcr=10 mL/min to 24 mL/min) for signs and symptoms of toxicity.
Cyclophosphamide and its metabolites are dialyzable although there are probably quantitative differences depending upon the dialysis system being used. Use of a consistent interval between cyclophosphamide administration and dialysis should be considered in patients requiring dialysis.
8.7. Hepatic Impairment
Patients with severe hepatic impairment have reduced conversion of cyclophosphamide to the active 4- hydroxyl metabolite, potentially reducing efficacy [see Clinical Pharmacology (12.3)].
The alcohol content of Cyclophosphamide Injection should be taken into account when given to patients with hepatic impairment [see Warnings and Precautions (5.7)].
-
10 OVERDOSAGE
No specific antidote for cyclophosphamide is known.
Overdosage should be managed with supportive measures, including appropriate treatment for any concurrent infection, myelosuppression, or cardiac toxicity should it occur.
Serious consequences of overdosage include manifestations of dose dependent toxicities such as myelosuppression, urotoxicity, cardiotoxicity (including cardiac failure), veno-occlusive hepatic disease, and stomatitis [see Warnings and Precautions (5.1, 5.2, 5.3, and 5.6)].
Patients who received an overdose should be closely monitored for the development of toxicities, and hematologic toxicity in particular.
Cyclophosphamide and its metabolites are dialyzable. Therefore, rapid hemodialysis is indicated when treating any suicidal or accidental overdose or intoxication.
Cystitis prophylaxis with mesna may be helpful in preventing or limiting urotoxic effects with cyclophosphamide overdose.
-
11 DESCRIPTION
Cyclophosphamide is an alkylating drug. It is an antineoplastic drug chemically related to the nitrogen mustards. The chemical name for cyclophosphamide is 2-[bis(2-chloroethyl)amino]tetrahydro-2H-1,3,2-oxazaphosphorine 2-oxide monohydrate, and has the following structural formula:
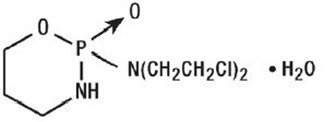
Cyclophosphamide has a molecular formula of C7H15Cl2N2O2P•H2O and a molecular weight of 279.1 g/mol. Cyclophosphamide is soluble in water, saline, or ethanol.
Cyclophosphamide Injection is a 200 mg/mL sterile clear colorless solution for intravenous use and is available as 500 mg and 1,000 mg strength vials.
- 500 mg vial contains 534.5 mg cyclophosphamide monohydrate equivalent to 500 mg cyclophosphamide and 83.9% (v/v) dehydrated alcohol.
- 1,000 mg vial contains 1,069 mg cyclophosphamide monohydrate equivalent to 1,000 mg cyclophosphamide and 83.9% (v/v) dehydrated alcohol.
-
12 CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
The mechanism of action has not been fully characterized. However, cross-linking of tumor cell DNA may be involved.
The active alkylating metabolites of cyclophosphamide interfere with the growth of susceptible rapidly proliferating malignant cells.12.2. Pharmacodynamics
Cyclophosphamide exposure-response relationships and the time course of pharmacodynamic response have not been fully characterized.
12.3. Pharmacokinetics
Pharmacokinetics are linear over the dose range used clinically.
Distribution
Cyclophosphamide volume of distribution approximates total body water (30 to 50 L). Cyclophosphamide is approximately 20% protein bound, with no dose dependent changes. Some metabolites are greater than 60% protein bound.
Elimination
The cyclophosphamide elimination half-life ranges from 3 to 12 hours with total body clearance (CL) values of 4 to 5.6 L/h following IV administration. Cyclophosphamide appears to induce its own metabolism. This auto-induction results in an increase in the total clearance, increased formation of active 4-hydroxyl metabolites and shortened elimination half-life values following repeated administration at 12- to 24-hour interval.
When cyclophosphamide was administered at 4.0 g/m2 (approximately 2 times the approved recommended dosage) over a 90-minutes infusion, saturable elimination in parallel with first-order renal elimination describe the kinetics of the drug.
Metabolism
The liver is the major site of cyclophosphamide activation. Approximately 75% of the administered dose of cyclophosphamide is activated by hepatic microsomal cytochrome P450s including CYP2A6, 2B6, 3A4, 3A5, 2C9, 2C18 and 2C19, with 2B6 displaying the highest 4-hydroxylase activity.
Cyclophosphamide is activated to form 4-hydroxycyclophosphamide, which is in equilibrium with its ring-open tautomer aldophosphamide. 4-hydroxycyclophosphamide and aldophosphamide can undergo further oxidation by aldehyde dehydrogenases to form the inactive metabolites 4-ketocyclophosphamide and carboxyphosphamide, respectively. Aldophosphamide can undergo β-elimination to form active metabolites phosphoramide mustard and acrolein. This spontaneous conversion can be catalyzed by albumin and other proteins. Less than 5% of cyclophosphamide may be directly detoxified by side chain oxidation, leading to the formation of inactive metabolites 2-dechloroethylcyclophosphamide. At high doses, the fraction of parent compound cleared by 4-hydroxylation is reduced resulting in non-linear elimination of cyclophosphamide in patients.
Excretion
Cyclophosphamide is primarily excreted as metabolites. 10 to 20% is excreted unchanged in the urine and 4% is excreted in the bile following IV administration.
Specific Populations
Renal Impairment
Cyclophosphamide exposure increased as the renal function decreased following one-hour intravenous infusion to renally impaired patients. Mean dose-corrected cyclophosphamide AUC increased by 38% in the moderate renal group, (Creatinine clearance (CLcr of 25 to 50 mL/min), by 64% in the severe renal group (CLcr of 10 to 24 mL/min) and by 23% in the hemodialysis group (CLcr of < 10mL/min) compared to the control group.
Cyclophosphamide is dialyzable. Dialysis clearance calculated by arterial-venous difference and actual drug recovery in dialysate averaged 104 mL/min, which is in the range of the metabolic clearance of 95 mL/min for the drug. A mean of 37% of the administered dose of cyclophosphamide was removed during hemodialysis. The elimination half- life (t1/2) was 3.3 hours in patients during hemodialysis, a 49% reduction of the 6.5 hours to the elimination half-life reported in uremic patients.
Hepatic Impairment
Total body clearance (CL) of cyclophosphamide is decreased by 40% in patients with severe hepatic impairment and elimination half-life (t½) is prolonged by 64%. Mean CL and t½ were 45 ± 8.6 L/kg and 12.5 ± 1.0 hours respectively, in patients with severe hepatic impairment and 63 ± 7.6 L/kg and 7.6 ± 1.4 hours respectively in the control group.
-
13 NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Cyclophosphamide administered by different routes, including intravenous, subcutaneous or intraperitoneal injection, or in drinking water, caused tumors in both mice and rats. In addition to leukemia and lymphoma, benign and malignant tumors were found at various tissue sites, including urinary bladder, mammary gland, lung, liver, and injection site[see Warnings and Precautions (5.5)].
Cyclophosphamide was mutagenic and clastogenic in multiple in vitro and in vivo genetic toxicology studies.
Cyclophosphamide is genotoxic in male and female germ cells. Animal data indicate that exposure of oocytes to cyclophosphamide during follicular development may result in a decreased rate of implantations and viable pregnancies, and in an increased risk of malformations. Male mice and rats treated with cyclophosphamide show alterations in male reproductive organs (e.g., decreased weights, atrophy, changes in spermatogenesis), and decreases in reproductive potential (e.g., decreased implantations and increased post-implantation loss) and increases in fetal malformations when mated with untreated females [see Use in Specific Populations (8.3)].
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Cyclophosphamide Injection is a 200 mg/mL clear or colorless ready-to-dilute sterile solution containing cyclophosphamide, USP.
Cyclophosphamide Injection
NDC Number
Presentation
Pack Factor
82943-100-03
500 mg per 2.5 mL Multiple-Dose Vial
1 vial per carton
82943-101-05
1,000 mg per 5 mL Multiple-Dose Vial
1 vial per carton
The container closure is not made with natural rubber latex.
Store the vials refrigerated at 2°C to 8°C (36°F to 46°F).
Cyclophosphamide is a hazardous product. Follow special handling and disposal procedures.1
-
17 PATIENT COUNSELING INFORMATION
Advise the patient of the following:
Myelosuppression, Immunosuppression, and Infections
Inform patients of the possibility of myelosuppression, immunosuppression, and infections. Explain the need for routine blood cell counts. Instruct patients to monitor their temperature frequently and immediately report any occurrence of fever [see Warnings and Precautions (5.1)].
Urinary Tract and Renal Toxicity
Advise the patient to report urinary symptoms (patients should report if their urine has turned a pink or red color) and the need for increasing fluid intake and frequent voiding [see Warnings and Precautions (5.2)].
Cardiotoxicity
Advise patients to contact a health care professional immediately for any of the following: new onset or worsening shortness of breath, cough, swelling of the ankles/legs, palpitations, weight gain of more than 5 pounds in 24 hours, dizziness or loss of consciousness [see Warnings and Precautions (5.3)].
Pulmonary Toxicity
Warn patients of the possibility of developing non-infectious pneumonitis. Advise patients to report promptly any new or worsening respiratory symptoms [see Warnings and Precautions (5.4)].
Alcohol Content
Explain to patients the possible effects of the alcohol content in Cyclophosphamide Injection, including possible effects on central nervous system. Patients in whom alcohol should be avoided or minimized should consider the alcohol content of Cyclophosphamide Injection. Alcohol could impair their ability to drive or use machines immediately after infusion [see Warnings and Precautions (5.7)].
Embryo-Fetal Toxicity
Inform female patients of the risk to a fetus and potential loss of the pregnancy. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warnings and Precautions (5.8)and Use in Specific Populations (8.1)].
Advise females of reproductive potential to use effective contraception during treatment and for up to 1 year after completion of therapy [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1, 8.3)].
Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 4 months after completion of therapy [see Warnings and Precautions (5.8) and Use in Specific Populations (8.1,8.3)].
Lactation
Advise lactating women not to breastfeed during treatment and for 1 week after the last dose of Cyclophosphamide Injection [see Use in Specific Populations (8.2)].
Infertility
Advise males and females of reproductive potential that Cyclophosphamide Injection may impair fertility [see Warnings and Precautions (5.9) and Use in Specific Populations (8.3, 8.4)].
Common Adverse Reactions
Explain to patients that side effects such as nausea, vomiting, stomatitis, impaired wound healing, amenorrhea, premature menopause, sterility and hair loss may be associated with cyclophosphamide administration. Other undesirable effects (including, e.g., dizziness, blurred vision, visual impairment) could affect the ability to drive or use machines [see Adverse Reactions (6.1)].
Distributed by:
Nevakar Injectables, Inc.
Bridgewater, NJ 08807
I07/2022 OSXXXX-01-90-XX
-
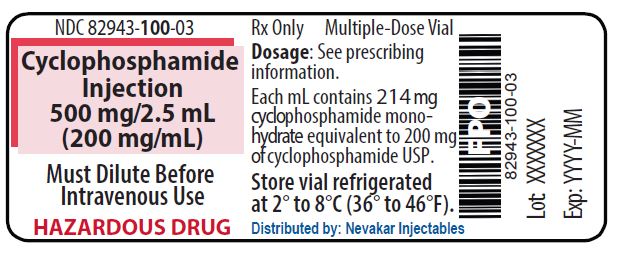
PRINCIPAL DISPLAY PANEL - 500 mg/2.5 mL Cyclophosphamide Injection
NDC 82943-100-03
Rx Only
Cyclophosphamide Injection
500 mg/2.5 mL
(200 mg/mL)Must Dilute Before Intravenous Use
HAZARDOUS DRUG
Multiple-Dose Vial
Each mL contains 214 mg cyclophosphamide monohydrate equivalent to 200 mg of cyclophosphamide USP
Store vial refrigerated
at 2° to 8°C (36° to 46°F)
Distributed by: Nevakar Injectables
Bridgewater, NJ 08807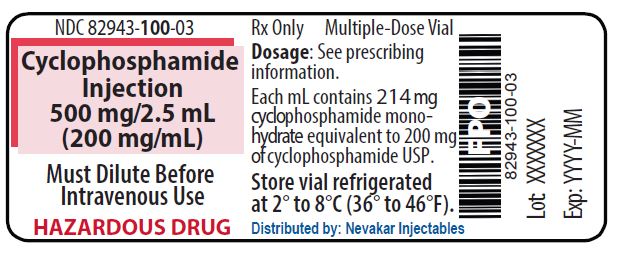
-
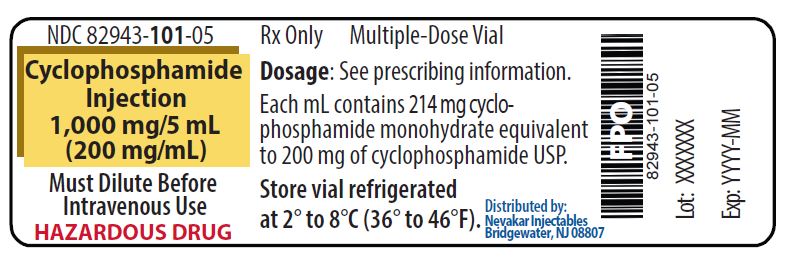
PRINCIPAL DISPLAY PANEL - 1,000 mg/5 mL Cyclophosphamide Injection
NDC 82943-101-05
Rx Only
Cyclophosphamide Injection
1,000 mg/5 mL
(200 mg/mL)Must Dilute Before Intravenous Use
HAZARDOUS DRUG
Multiple-Dose Vial
Each mL contains 214 mg cyclophosphamide monohydrate equivalent to 200 mg of cyclophosphamide USP
Store vial refrigerated
at 2° to 8°C (36° to 46°F)Distributed by: Nevakar Injectables
Bridgewater, NJ 08807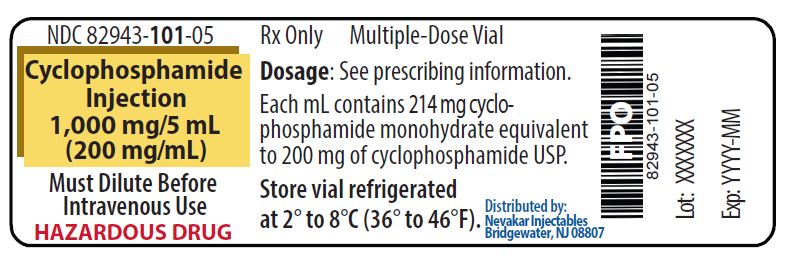
-
INGREDIENTS AND APPEARANCE
CYCLOPHOSPHAMIDE
cyclophosphamide injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82943-100 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOPHOSPHAMIDE (UNII: 8N3DW7272P) (CYCLOPHOSPHAMIDE ANHYDROUS - UNII:6UXW23996M) CYCLOPHOSPHAMIDE ANHYDROUS 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) NITROGEN (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82943-100-03 1 in 1 CARTON 01/01/2024 1 2.5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217651 01/01/2024 CYCLOPHOSPHAMIDE
cyclophosphamide injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:82943-101 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOPHOSPHAMIDE (UNII: 8N3DW7272P) (CYCLOPHOSPHAMIDE ANHYDROUS - UNII:6UXW23996M) CYCLOPHOSPHAMIDE ANHYDROUS 200 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) NITROGEN (UNII: N762921K75) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82943-101-05 1 in 1 CARTON 01/01/2024 1 5 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA217651 01/01/2024 Labeler - Nevakar Injectables Inc. (118160065)