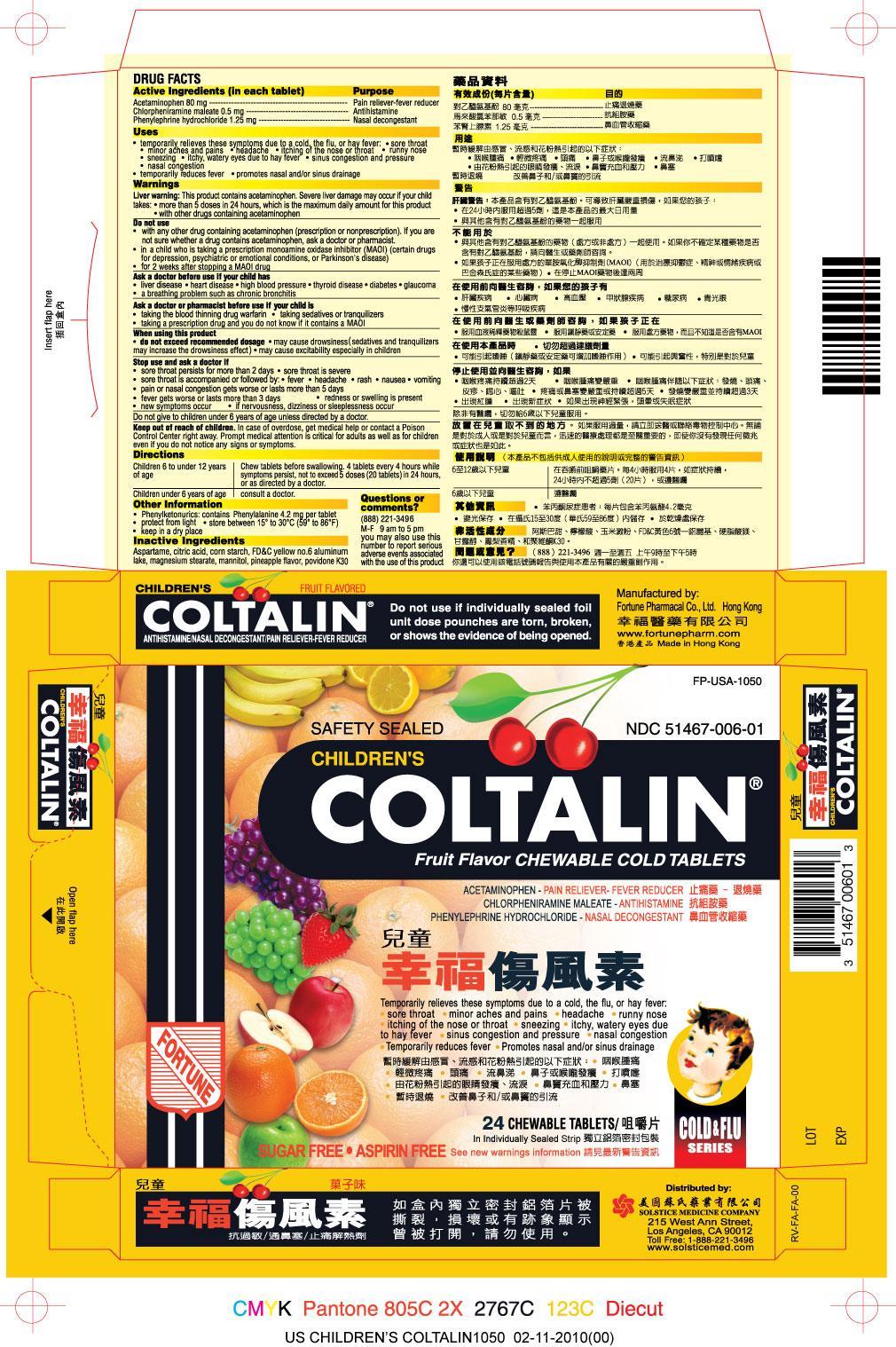
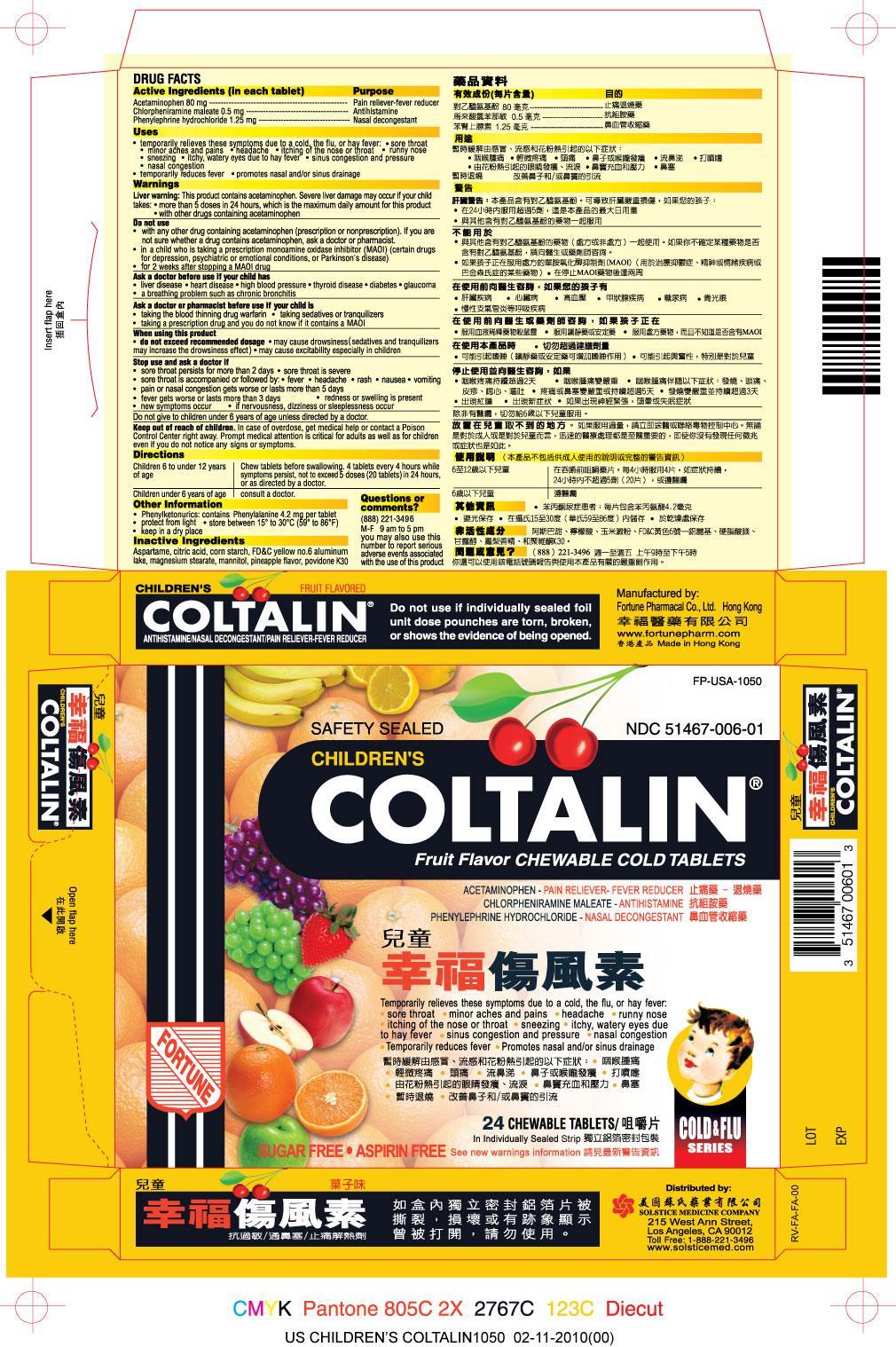
Label: CHILDRENS COLTALIN- acetaminophen, chlorpheniramine maleate, phenylephrine hydrochloride tablet, chewable
- NDC Code(s): 51467-006-01
- Packager: FORTUNE PHARMACAL COMPANY LIMITED
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 13, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
-
INDICATIONS & USAGE
Uses
temporarily relieves these symptoms due to a cold, the flu, or hay fever:
sore throat
minor aches and pains
headache
itching of the nose or throat
runny nose
sneezing itchy, watery eyes due to hay fever
sinus congestion and pressure
nasal congestion
temporarily reduces fever
promotes nasal and/or sinus drainage - WARNINGS
-
DO NOT USE
Do not use
with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease). If you do not know whether a prescription drug contains an MAOI, ask a doctor or pharmacist.
for 2 weeks after stopping the MAOI drug - ASK DOCTOR
- ASK DOCTOR/PHARMACIST
- WHEN USING
-
STOP USE
Stop use and ask a doctor if
sore throat persists for more than 2 days
sore throat is severe
sore throat is accompanied or followed by: fever, headache, rash, nausea, vomitting
pain or nasal congestion gets worse or lasts more than 5 day
fever gets worse or lasts more than 3 days
redness or swelling is presen
new symptoms occur
if nervousness, dizziness, or sleeplessness occur - PREGNANCY OR BREAST FEEDING
-
KEEP OUT OF REACH OF CHILDREN
Do not give to children under 6 years of age unless directed by a doctor.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as children even if you do not notice any signs or symptoms.
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- QUESTIONS
-
PRINCIPAL DISPLAY PANEL
Children's Coltalin
NDC 51467-006-01
Acetaminophen - Pain Reliever-Fever Reducer
Chlorpheniramine maleate - Antihistamine
Phenylephrine hydrochloride- DecongestantSee new warnings information
Sugar Free
Aspirin Free
24 Chewable Tablets
Safety Sealed
Do not use if individually sealed foil unit dose pouches are torn, broken, or shows the evidence of being opened.
-
INGREDIENTS AND APPEARANCE
CHILDRENS COLTALIN
acetaminophen, chlorpheniramine maleate, phenylephrine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51467-006 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 80 mg CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 0.5 mg PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 1.25 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) STARCH, CORN (UNII: O8232NY3SJ) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) PINEAPPLE (UNII: 2A88ZO081O) POVIDONE K30 (UNII: U725QWY32X) Product Characteristics Color orange Score no score Shape ROUND Size 12mm Flavor PINEAPPLE Imprint Code F;O;R;T;U;N;E Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51467-006-01 24 in 1 CARTON; Type 1: Convenience Kit of Co-Package 03/01/2004 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 03/01/2004 Labeler - FORTUNE PHARMACAL COMPANY LIMITED (686280561) Establishment Name Address ID/FEI Business Operations FORTUNE PHARMACAL COMPANY LIMITED 686280561 manufacture(51467-006)