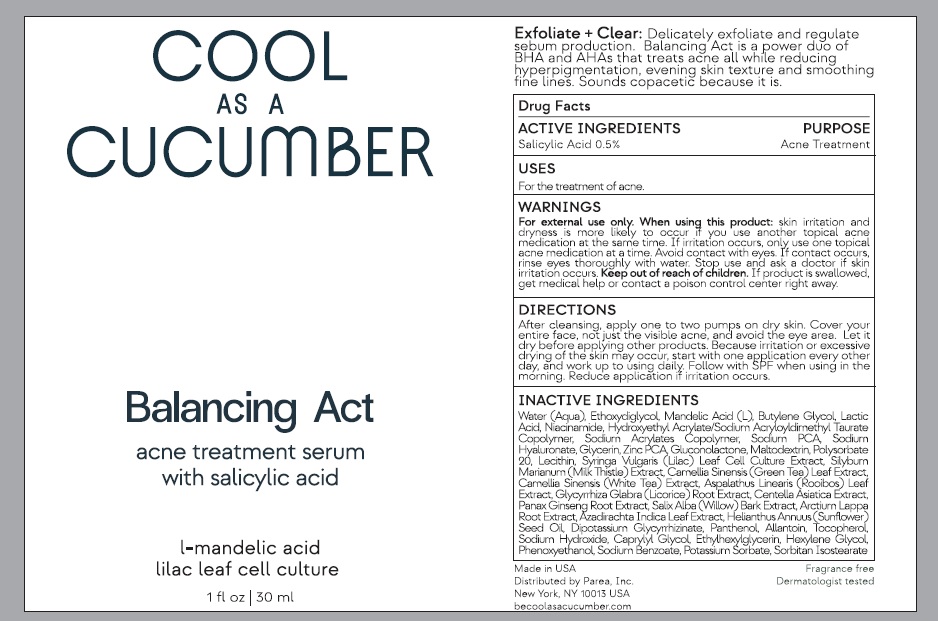
Label: BALANCING ACT- salicylic acid liquid
- NDC Code(s): 83360-001-01
- Packager: Parea, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only.
When using this product : skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If
irritation occurs, only use one topical acne medication at a time. Avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water.
Stop use and ask doctor if skin irritation occurs.
Keep out of reach of children. If product is swallowed get medical help or contact a poison control center right away.
- KEEP OUT OF REACH OF CHILDREN
-
Direction
After cleansing, apply one to two pumps on dry skin. Cover your entire face, not just the visible acne, and avoid the eye area. Let it dry before applying other products. Because irritation or excessive drying of the skin may occur, start with one application every other day, and work up to using daily. Follow with SPF when using in the morning. Reduce application if irritation occurs.
-
Inactive ingredients
water, diethylene glycol monoethyl ether, mandelic acid, butylene glycol, lactic acid, unspecified form, niacinamide, hydroxyethyl acrylate/sodium acryloyldimethyl taurate copolymer(100000 mpa.s at 1.5%), sodium acrylates crosspolymer-2, sodium pyrrolidone carboxylate, hyaluronate sodium, glycerin, zinc pidolate, gluconolactone, maltodextrin, polysorbate 20, lecithin, soybean, syringa vulgaris pollen, milk thistle, green tea leaf, white tea, aspalathus linearis leaf, glycyrrhiza glabra, centella asiatica triterpenoids, asian ginseng, salix alba bark, arctium lappa root, azadirachta indica leaf, sunflower oil, glycyrrhizinate dipotassium, panthenol, allantoin, tocopherol, sodium hydroxide, caprylyl glycol, ethylhexylglycerin, hexylene glycol, phenoxyethanol, sodium benzoate, potassium sorbate, sorbitan isostearateonic
- Other information
- Product label
-
INGREDIENTS AND APPEARANCE
BALANCING ACT
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83360-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 0.5 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) MANDELIC ACID (UNII: NH496X0UJX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) NIACINAMIDE (UNII: 25X51I8RD4) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) SODIUM ACRYLATES CROSSPOLYMER-2 (UNII: D3HPR4WW6F) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GLYCERIN (UNII: PDC6A3C0OX) ZINC PIDOLATE (UNII: C32PQ86DH4) GLUCONOLACTONE (UNII: WQ29KQ9POT) MALTODEXTRIN (UNII: 7CVR7L4A2D) POLYSORBATE 20 (UNII: 7T1F30V5YH) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SYRINGA VULGARIS POLLEN (UNII: 2BO2QL8OR1) MILK THISTLE (UNII: U946SH95EE) GREEN TEA LEAF (UNII: W2ZU1RY8B0) WHITE TEA (UNII: O0M3396E09) ASPALATHUS LINEARIS LEAF (UNII: H7UGK1GJCU) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) CENTELLA ASIATICA TRITERPENOIDS (UNII: 4YS74Q4G4J) ASIAN GINSENG (UNII: CUQ3A77YXI) SALIX ALBA BARK (UNII: 205MXS71H7) ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) AZADIRACHTA INDICA LEAF (UNII: HKY915780T) SUNFLOWER OIL (UNII: 3W1JG795YI) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) PANTHENOL (UNII: WV9CM0O67Z) ALLANTOIN (UNII: 344S277G0Z) TOCOPHEROL (UNII: R0ZB2556P8) SODIUM HYDROXIDE (UNII: 55X04QC32I) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) HEXYLENE GLYCOL (UNII: KEH0A3F75J) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM BENZOATE (UNII: OJ245FE5EU) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83360-001-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/06/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333D 04/06/2023 Labeler - Parea, Inc. (118943804)