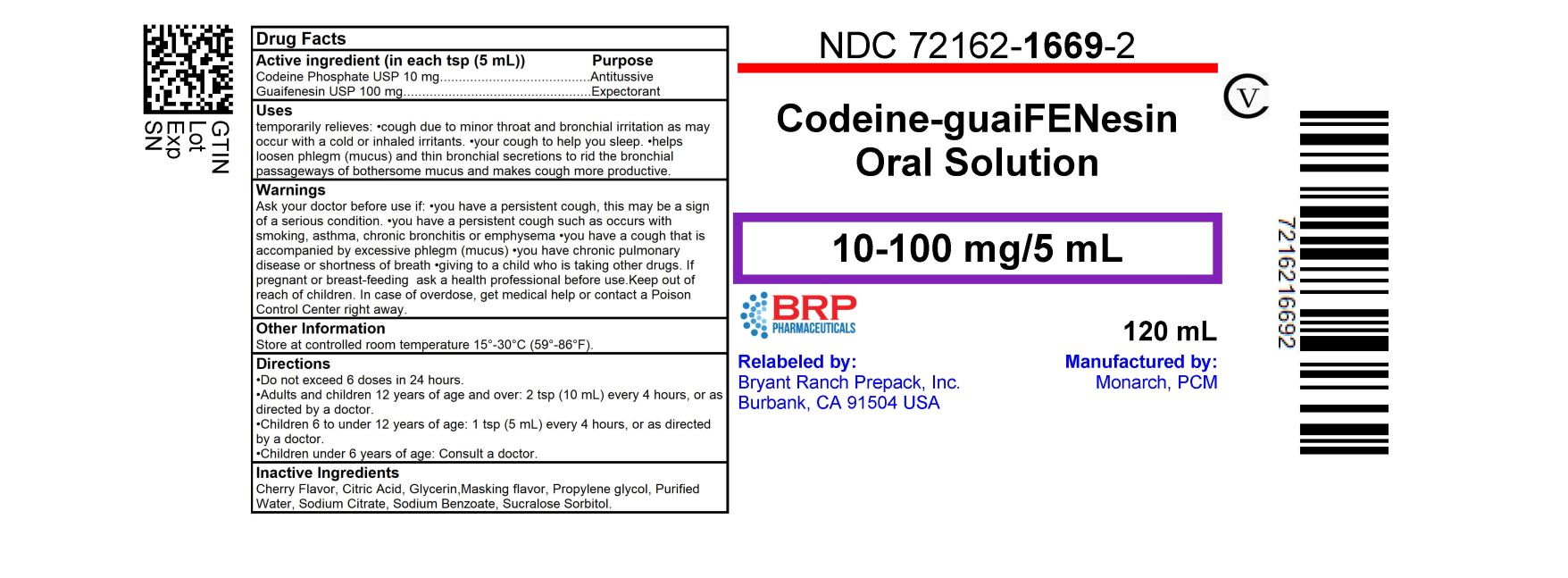
Label: CODEINE-GUAIFENESIN- codeine phosphate and guaifenesin solution
- NDC Code(s): 72162-1669-2
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 58657-500
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 22, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask your doctor before use if
- you have a persistent cough, this may be a sign of a serious condition
- you have a persistent cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- you have a cough that is accompanied by excessive phlegm (mucus)
- you have chronic pulmonary disease or shortness of breath
- giving to a child who is taking other drugs
When using this product
- giving a higher dose than recommended by a doctor could result in serious side effects for your child. A special measuring device should be used to give an accurate dose of this product to children under 6 years of age.
- may cause or aggravate constipation
- Directions
- Other information
- Inactive ingredients
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CODEINE-GUAIFENESIN
codeine phosphate and guaifenesin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72162-1669(NDC:58657-500) Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE ANHYDROUS - UNII:UX6OWY2V7J) CODEINE PHOSPHATE 10 mg in 5 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) SORBITOL (UNII: 506T60A25R) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72162-1669-2 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 03/22/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 04/01/2014 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(72162-1669) , RELABEL(72162-1669)