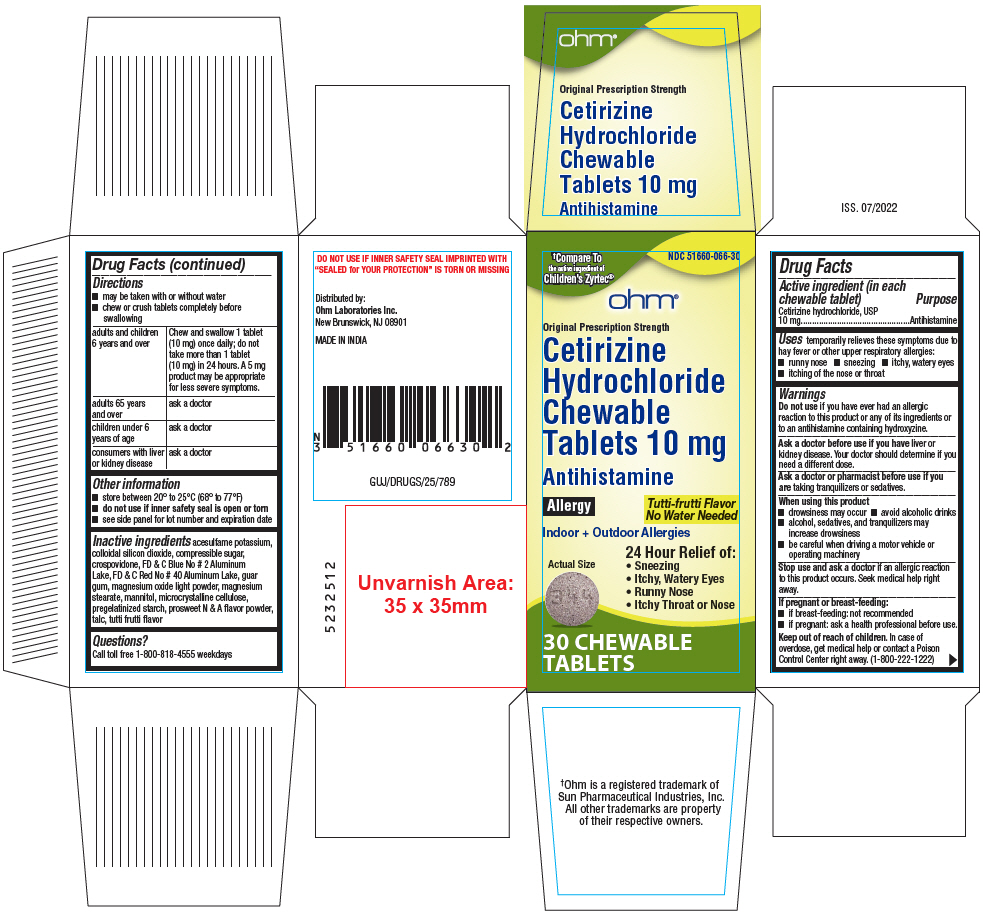
Label: CETIRIZINE HYDROCHLORIDE tablet, chewable
- NDC Code(s): 51660-066-30
- Packager: OHM LABORATORIES INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated July 26, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
-
Warnings
Do not use if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing hydroxyzine.
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if an allergic reaction to this product occurs. Seek medical help right away.
-
Directions
- may be taken with or without water
- chew or crush tablets completely before swallowing
adults and children 6 years and over Chew and swallow 1 tablet (10 mg) once daily; do not take more than 1 tablet (10 mg) in 24 hours. A 5 mg product may be appropriate for less severe symptoms. adults 65 years and over ask a doctor children under 6 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other information
-
Inactive ingredients
acesulfame potassium, colloidal silicon dioxide, compressible sugar, crospovidone, FD & C Blue No # 2 Aluminum Lake, FD & C Red No # 40 Aluminum Lake, guar gum, magnesium oxide light powder, magnesium stearate, mannitol, microcrystalline cellulose, pregelatinized starch, prosweet N & A flavor powder, talc, tutti frutti flavor
- Questions?
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Carton
†Compare To
the active ingredient of
Children's Zyrtec®NDC 51660-066-30
ohm®
Original Prescription Strength
Cetirizine
Hydrochloride
Chewable
Tablets 10 mgAntihistamine
Allergy
Tutti-frutti Flavor
No Water NeededIndoor + Outdoor Allergies
Actual Size
24 Hour Relief of:
- Sneezing
- Itchy, Watery Eyes
- Runny Nose
- Itchy Throat or Nose
30 CHEWABLE
TABLETS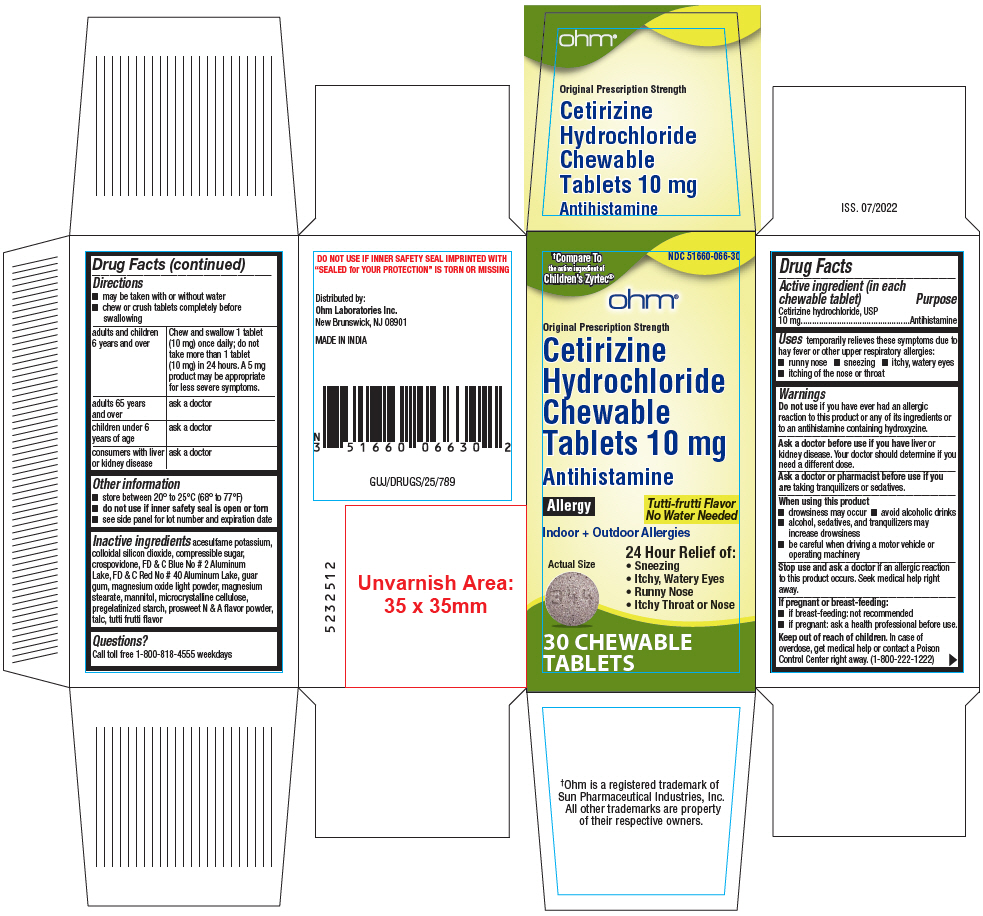
-
INGREDIENTS AND APPEARANCE
CETIRIZINE HYDROCHLORIDE
cetirizine hydrochloride tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51660-066 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CETIRIZINE HYDROCHLORIDE (UNII: 64O047KTOA) (CETIRIZINE - UNII:YO7261ME24) CETIRIZINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SUCROSE (UNII: C151H8M554) CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) GUAR GUM (UNII: E89I1637KE) MAGNESIUM OXIDE (UNII: 3A3U0GI71G) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color PURPLE Score no score Shape ROUND Size 10mm Flavor TUTTI FRUTTI Imprint Code 344 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51660-066-30 1 in 1 CARTON 07/21/2022 1 30 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090142 07/21/2022 Labeler - OHM LABORATORIES INC. (184769029) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries Limited 725959238 MANUFACTURE(51660-066)