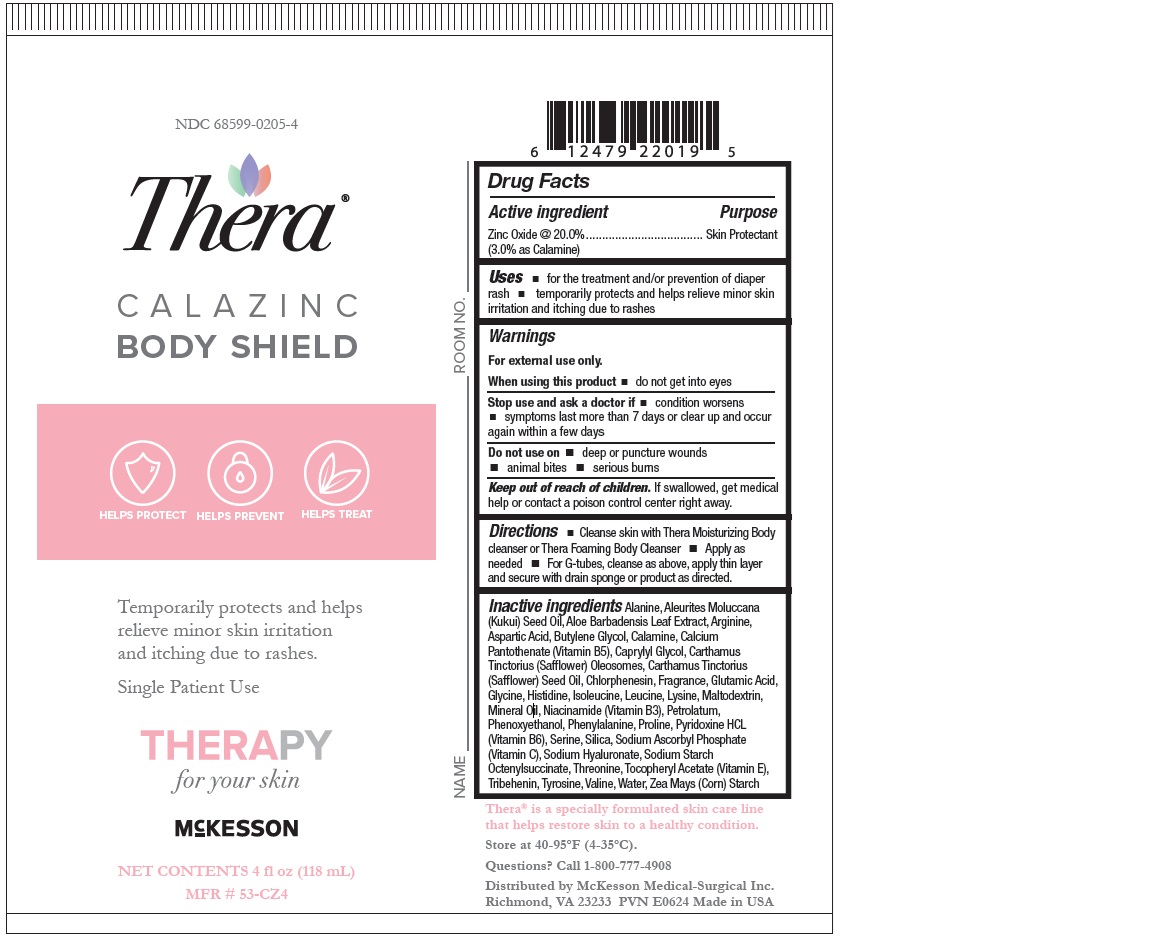
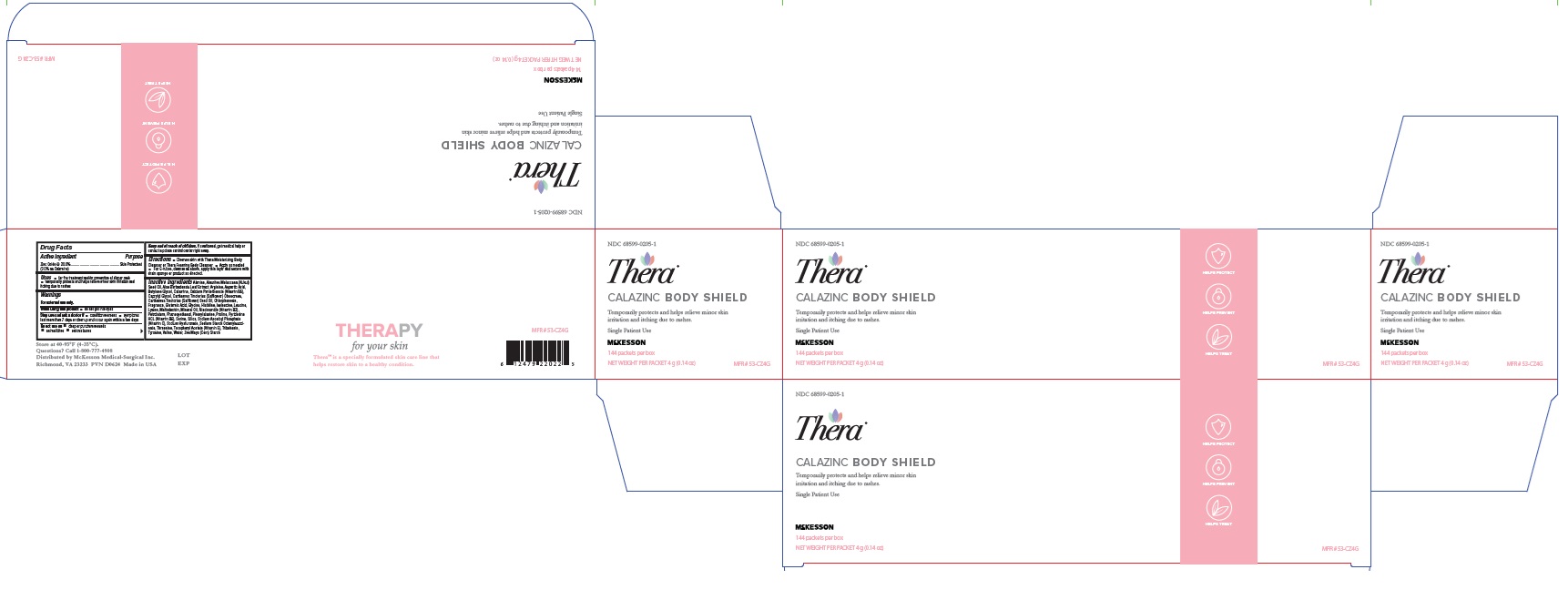
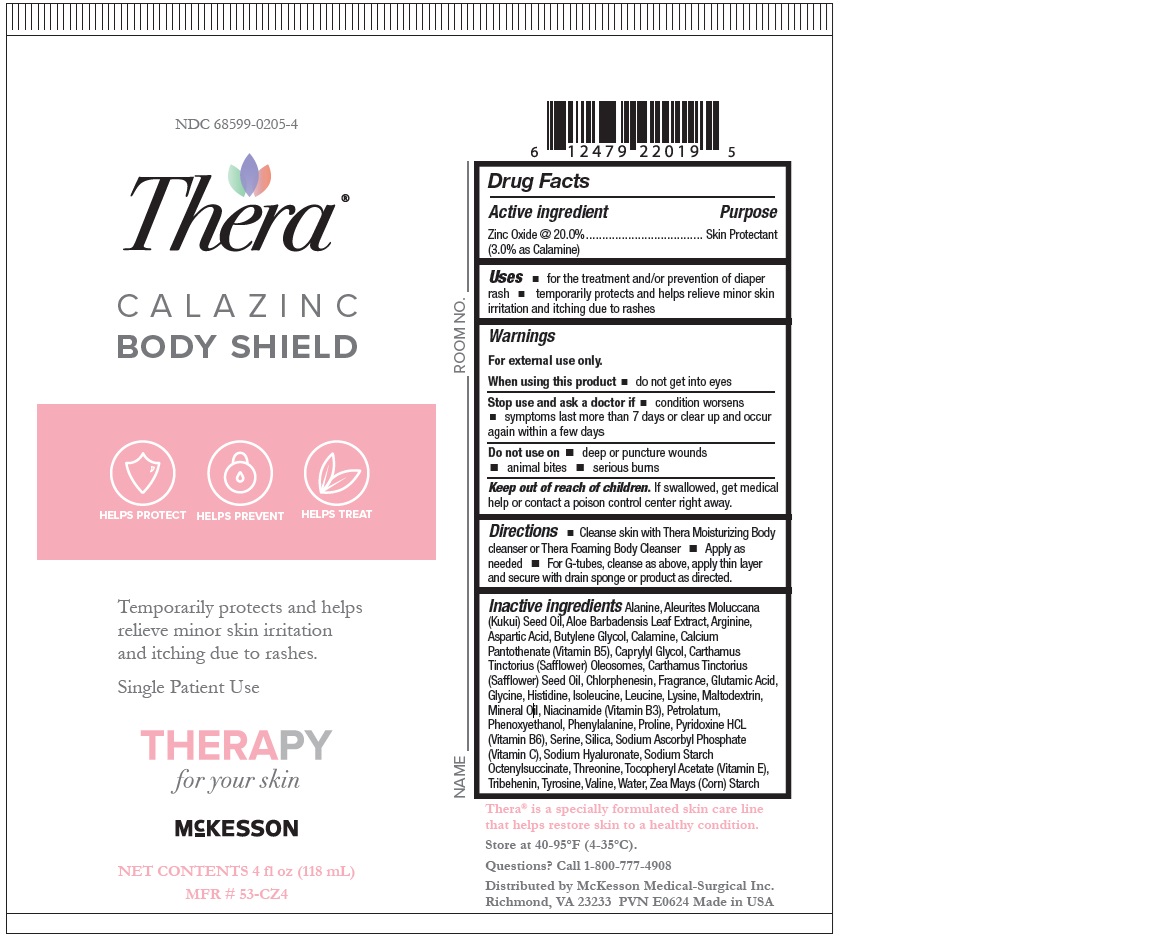
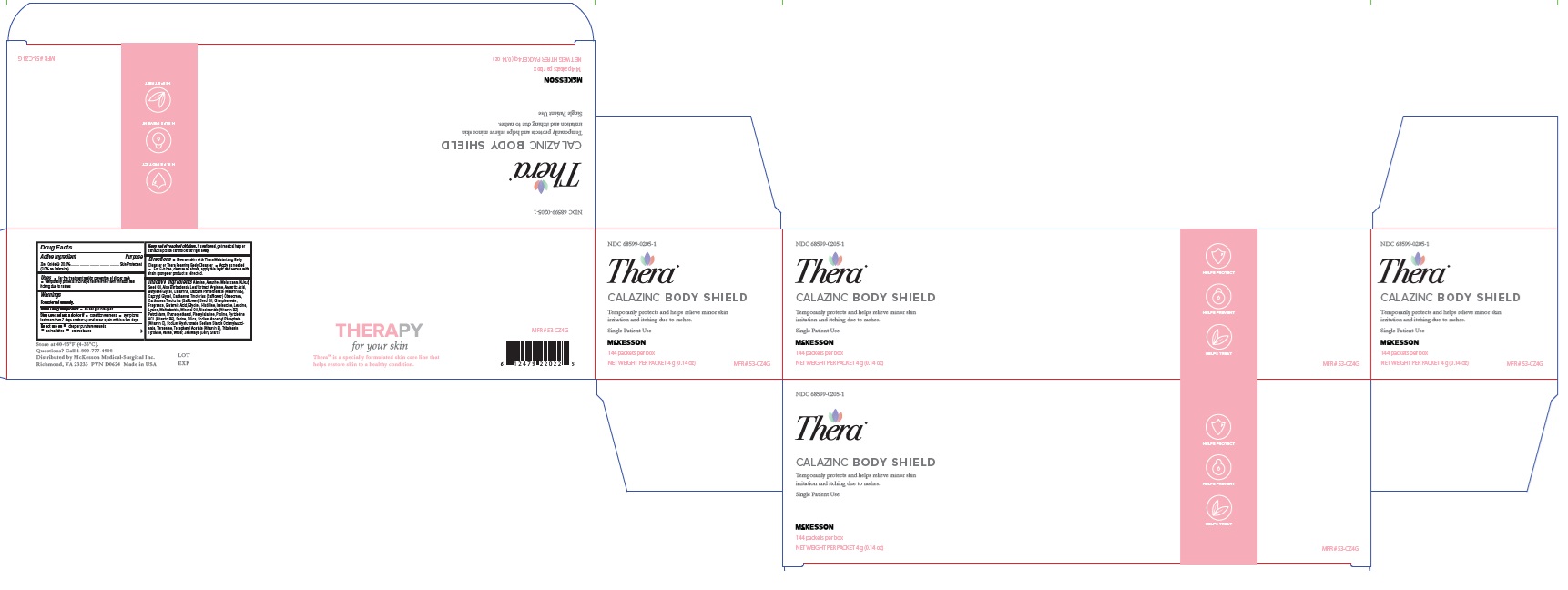
Label: THERA CALAZINC BODY SHIELD- zinc oxide paste
- NDC Code(s): 68599-0205-1, 68599-0205-4
- Packager: McKesson Medical-Surgical Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated June 17, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep out of reach of children.
- Uses
- Warnings
- Directions
- Other information
-
INACTIVE INGREDIENT
- Aleurites Moluccana Seed Oil,
- Aloe Barbadensis (Aloe Vera) Leaf Juice,
- SAFFLEX TM(Consisting of: Calcium Pantothenate (Vitamin B 5),
- Maltodexdrin,
- Niacinamide (Vitamin B 3),
- Pyridoxine HCl (Vitamin B 6),
- Silica,
- Sodium Ascorbyl Phosphate (Vitamin C),
- Sodium Starch Octenylsuccinate,
- Tocopheryl Acetate (Vitamin E)), Bisabolol,
- Carthamus Tinctorius (Safflower) Olesomes,
- Carthamus Tinctorius (Safflower) Seed Oil,
- Lavender Ylang Fragrance,
- Modified Corn Starch,
- Pentaerythrityl Tetra-di-t-Butyl Hydroxyhydrocinnamate,
- Petrolatum,
- Phenoxyethanol,
- Sodium Hyaluronate,
- Zingiber Officinale (Ginger) Root Extract.
- Label (4 oz)
- Label(4 g)
-
INGREDIENTS AND APPEARANCE
THERA CALAZINC BODY SHIELD
zinc oxide pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68599-0205 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 200 mg in 1 g Inactive Ingredients Ingredient Name Strength HISTIDINE (UNII: 4QD397987E) TYROSINE (UNII: 42HK56048U) LYSINE (UNII: K3Z4F929H6) ISOLEUCINE (UNII: 04Y7590D77) KUKUI NUT OIL (UNII: TP11QR7B8R) ALOE VERA LEAF (UNII: ZY81Z83H0X) CALCIUM PANTOTHENATE (UNII: 568ET80C3D) MALTODEXTRIN (UNII: 7CVR7L4A2D) NIACINAMIDE (UNII: 25X51I8RD4) PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) LEVOMENOL (UNII: 24WE03BX2T) SAFFLOWER OIL (UNII: 65UEH262IS) PETROLATUM (UNII: 4T6H12BN9U) HYALURONATE SODIUM (UNII: YSE9PPT4TH) GINGER (UNII: C5529G5JPQ) ASPARTIC ACID (UNII: 30KYC7MIAI) ALANINE (UNII: OF5P57N2ZX) PROLINE (UNII: 9DLQ4CIU6V) MINERAL OIL (UNII: T5L8T28FGP) VALINE (UNII: HG18B9YRS7) WATER (UNII: 059QF0KO0R) SERINE (UNII: 452VLY9402) CHLORPHENESIN (UNII: I670DAL4SZ) OCTENYLSUCCINIC ACID (UNII: 12UZE4X73L) GLYCINE (UNII: TE7660XO1C) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) PHENYLALANINE (UNII: 47E5O17Y3R) ZEA MAYS WHOLE (UNII: 1G5HNE09V8) LEUCINE (UNII: GMW67QNF9C) ARGININE (UNII: 94ZLA3W45F) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ZINC CARBONATE (UNII: EQR32Y7H0M) THREONINE (UNII: 2ZD004190S) GLUTAMIC ACID (UNII: 3KX376GY7L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68599-0205-4 113 g in 1 TUBE; Type 0: Not a Combination Product 09/01/2011 2 NDC:68599-0205-1 144 in 1 CARTON 09/01/2011 2 4 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 09/01/2011 Labeler - McKesson Medical-Surgical Inc. (023904428) Establishment Name Address ID/FEI Business Operations Central Solutions 007118524 manufacture(68599-0205)