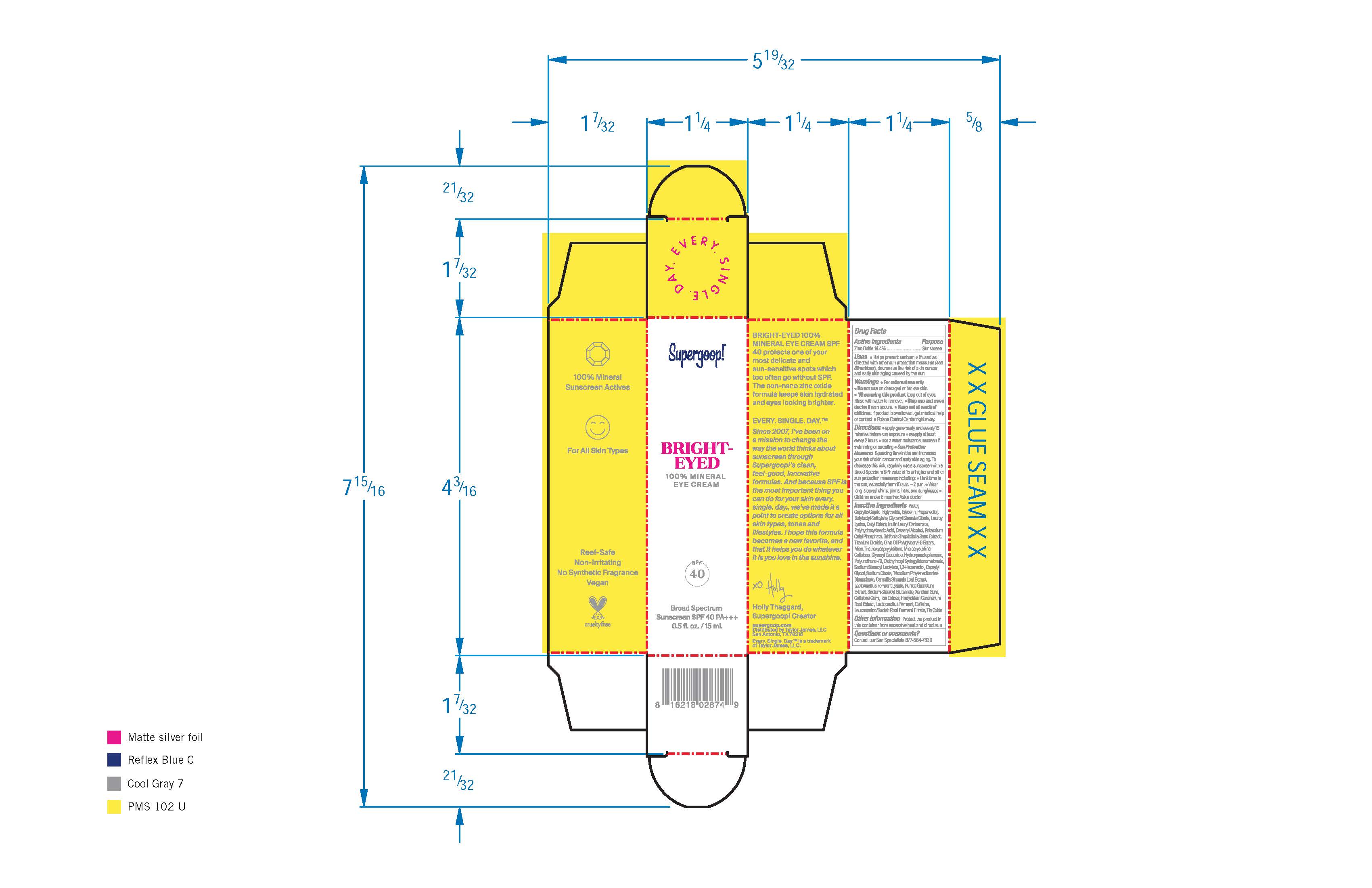
Label: BRIGHT-EYED 100% MINERAL EYE CREAM BROAD SPECTRUM SUNSCREEN SPF 40- zinc oxide cream
- NDC Code(s): 75936-149-01, 75936-149-02, 75936-149-03, 75936-149-04
- Packager: Supergoop, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated July 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
INACTIVE INGREDIENT
Inactive Ingredients Water, Caprylic/Capric Triglyceride, Glycerin, Propanediol, Butyloctyl Salicylate, Glyceryl Stearate Citrate, Lauroyl Lysine, Cetyl Esters, Inulin Lauryl CarbamatePolyhydroxystearic Acid, Cetearyl Alcohol, Potassium Cetyl phosophate, Griffonia Simplicifolia Seed Extract, Titanium Dioxide, Olive Oil Polyglyceryl-6 Esters, Mica, Triethoxycaprylylsilane, Microcrystalline cellulose, Glyceryl Glucoside, Hydroxyacetophenone, Polyurethane-79, Diethylhexyl Syringylidenemalonate, Sodium Stearoyl Lactylate, 1,2-Hexanediol, Caprylyl Glycol, Sodium Citrate, Trisodium Ethylenediamine Disuccinate, Camellia Sinensis Leaf Extract, Lactobacillus Ferment Lysate, Punica Granatum Extract, Sodium Citrate, Sodium Stearoyl Glutamate, Xanthan Gum, Cellulose Gum, Iron Oxides, Hedychium Coronarium Root Extract, Lactobacillus Ferment, Caffeine, Leuconostoc/Radish Root Ferment Filtrate, Tin Oxide.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BRIGHT-EYED 100% MINERAL EYE CREAM BROAD SPECTRUM SUNSCREEN SPF 40
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-149 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 14.4 g in 100 mL Inactive Ingredients Ingredient Name Strength LACTOBACILLUS REUTERI (UNII: 9913I24QEE) CAFFEINE (UNII: 3G6A5W338E) GLYCERYL STEARATE CITRATE (UNII: WH8T92A065) LAUROYL LYSINE (UNII: 113171Q70B) PUNICA GRANATUM ROOT BARK (UNII: CLV24I3T1D) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CAPRYLYL GLYCOL (UNII: 00YIU5438U) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) GREEN TEA LEAF (UNII: W2ZU1RY8B0) XANTHAN GUM (UNII: TTV12P4NEE) LEUCONOSTOC/RADISH ROOT FERMENT FILTRATE (UNII: D2QHA03458) FERRIC OXIDE RED (UNII: 1K09F3G675) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) GRIFFONIA SIMPLICIFOLIA SEED (UNII: LUS5142TMY) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) SODIUM STEAROYL LACTYLATE (UNII: IN99IT31LN) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) MICA (UNII: V8A1AW0880) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SODIUM STEAROYL GLUTAMATE (UNII: 65A9F4P024) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PROPANEDIOL (UNII: 5965N8W85T) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CETYL ESTERS WAX (UNII: D072FFP9GU) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) STANNIC OXIDE (UNII: KM7N50LOS6) HEDYCHIUM CORONARIUM ROOT (UNII: 92A6N0IQN9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-149-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2020 2 NDC:75936-149-02 5 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2020 3 NDC:75936-149-03 1.5 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2020 4 NDC:75936-149-04 5 mL in 1 TUBE; Type 0: Not a Combination Product 01/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 01/01/2020 Labeler - Supergoop, LLC (117061743)