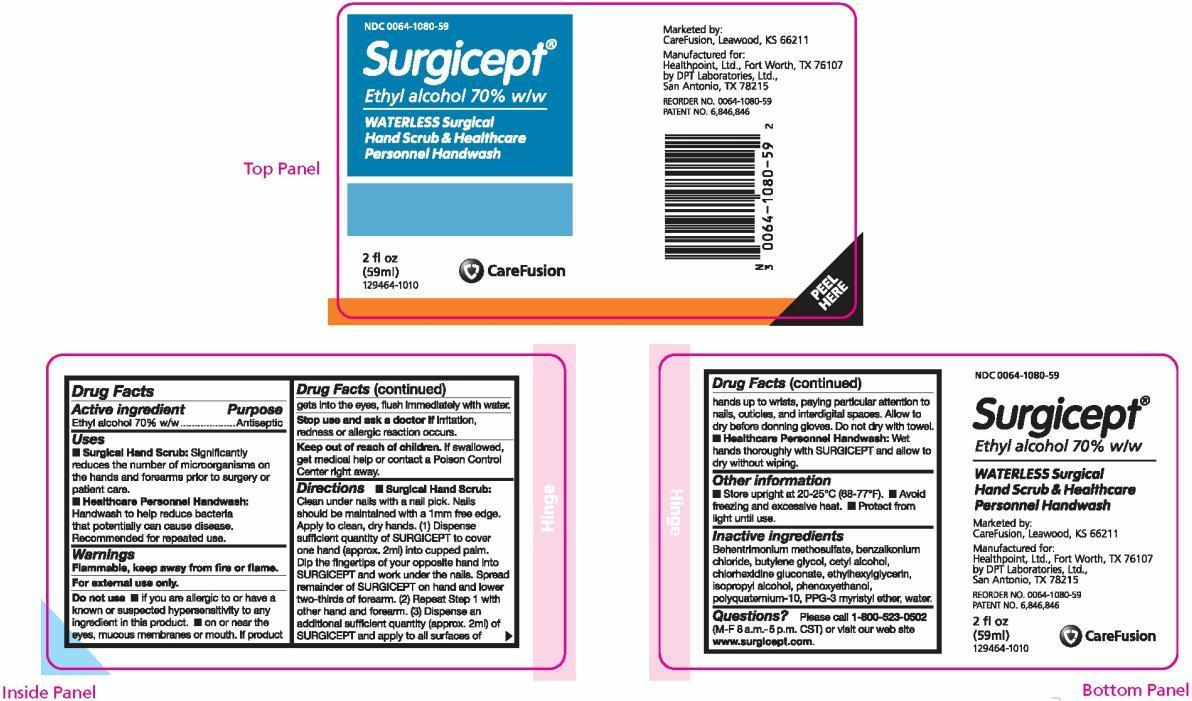
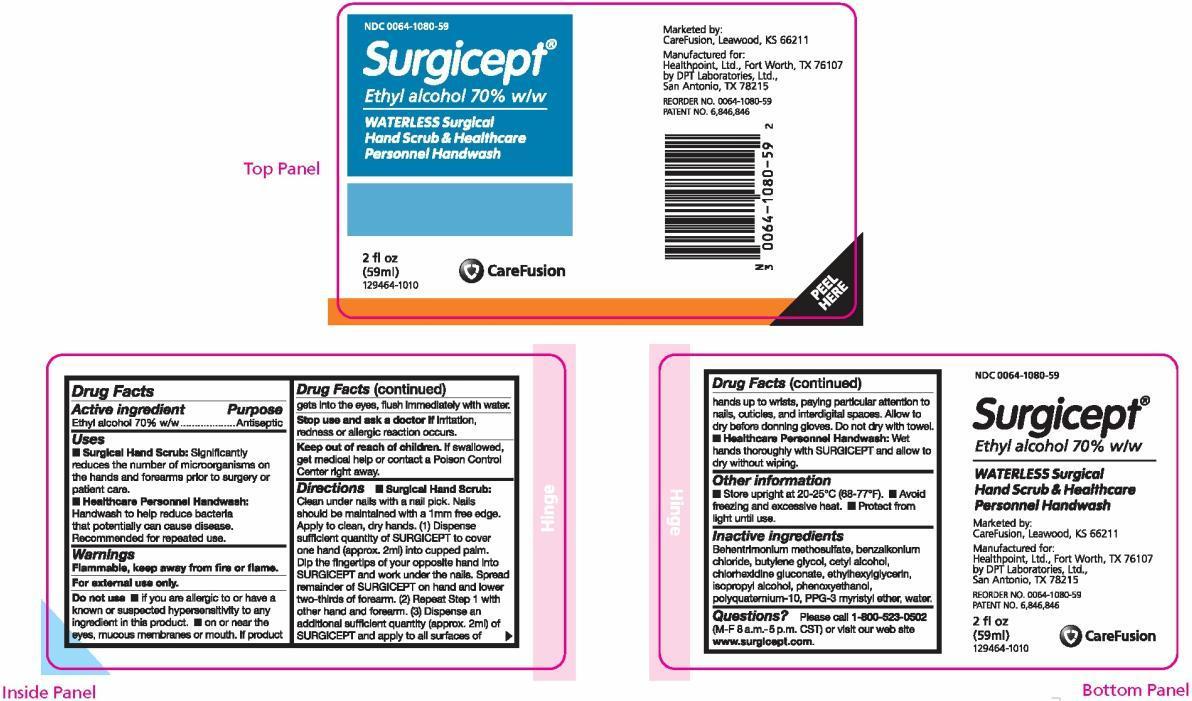
Label: SURGICEPT WATERLESS, SURGICAL HANDSCRUB AND HEALTHCARE PERSONNEL HANDWASH- ethyl alcohol solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 0064-1080-11, 0064-1080-59, 0064-1080-80 - Packager: HEALTHPOINT, LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 11, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
- Flammable, keep away from fire or flame.
- For external use only.
-
Do not use
- if you are allergic to or have a known or suspected hypersensitivity to any ingredient in this product.
- on or near the eyes, mucous membranes or mouth. If product gets into the eyes, flush immediately with water.
- Stop use and ask a doctor if irritation, redness or allergic reaction occurs.
- Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
-
Directions
- Surgical Hand Scrub: Clean under nails with a nail pick. Nails should be maintained with a 1mm free edge. Apply to clean, dry hands. (1) Dispense sufficient quantity of SURGICEPT to cover one hand (approx. 2ml) into cupped palm. Dip the fingertips of your opposite hand into SURGICEPT and work under the nails. Spread remainder of SURGICEPT on hand and lower two-thirds of forearm. (2) Repeat Step 1 with other hand and forearm. (3) Dispense an additional sufficient quantity (approx. 2ml) of SURGICEPT and apply to all surfaces of hands up to wrists, paying particular attention to nails, cuticles, and interdigital spaces. Allow to dry before donning gloves. Do not dry with a towel.
- Healthcare Personnel Handwash: Wet hands thoroughly with SURGICEPT and allow to dry without wiping.
- Inactive ingredients
-
Questions?
Please call 1-800-523-0502 (M-F 8 a.m.-5 p.m. CST) or visit our web site www.surgicept.com.
PRINCIPAL DISPLAY PANEL
NDC 0064-1080-59
Surgicept ®
Ethyl alcohol 70% w/w
WATERLESS Surgical Hand Scrub & Healthcare Personnel Handwash
2 fl oz (59 ml)
129464-1010Marketed by:
CareFusion, Leawood, KS 66211Manufactured for:
Healthpoint, Ltd., Fort Worth, TX 76107
by DPT Laboratories, Ltd., San Antonio, TX 78215REORDER NO. 0064-1080-59
PATENT NO. 6,846,846 - PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SURGICEPT WATERLESS, SURGICAL HANDSCRUB AND HEALTHCARE PERSONNEL HANDWASH
ethyl alcohol solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0064-1080 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.75 mL in 1 mL Inactive Ingredients Ingredient Name Strength BEHENTRIMONIUM METHOSULFATE (UNII: 5SHP745C61) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CETYL ALCOHOL (UNII: 936JST6JCN) CHLORHEXIDINE GLUCONATE (UNII: MOR84MUD8E) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ISOPROPYL ALCOHOL (UNII: ND2M416302) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYQUATERNIUM-10 (400 CPS AT 2%) (UNII: HB1401PQFS) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (CLEAR) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0064-1080-59 59 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2006 2 NDC:0064-1080-11 1150 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/01/2006 3 NDC:0064-1080-80 1 in 1 CARTON 01/01/2006 02/28/2015 3 800 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 01/01/2006 Labeler - HEALTHPOINT, LTD (965634504) Establishment Name Address ID/FEI Business Operations DPT LABORATORIES, LTD 832224526 manufacture(0064-1080)