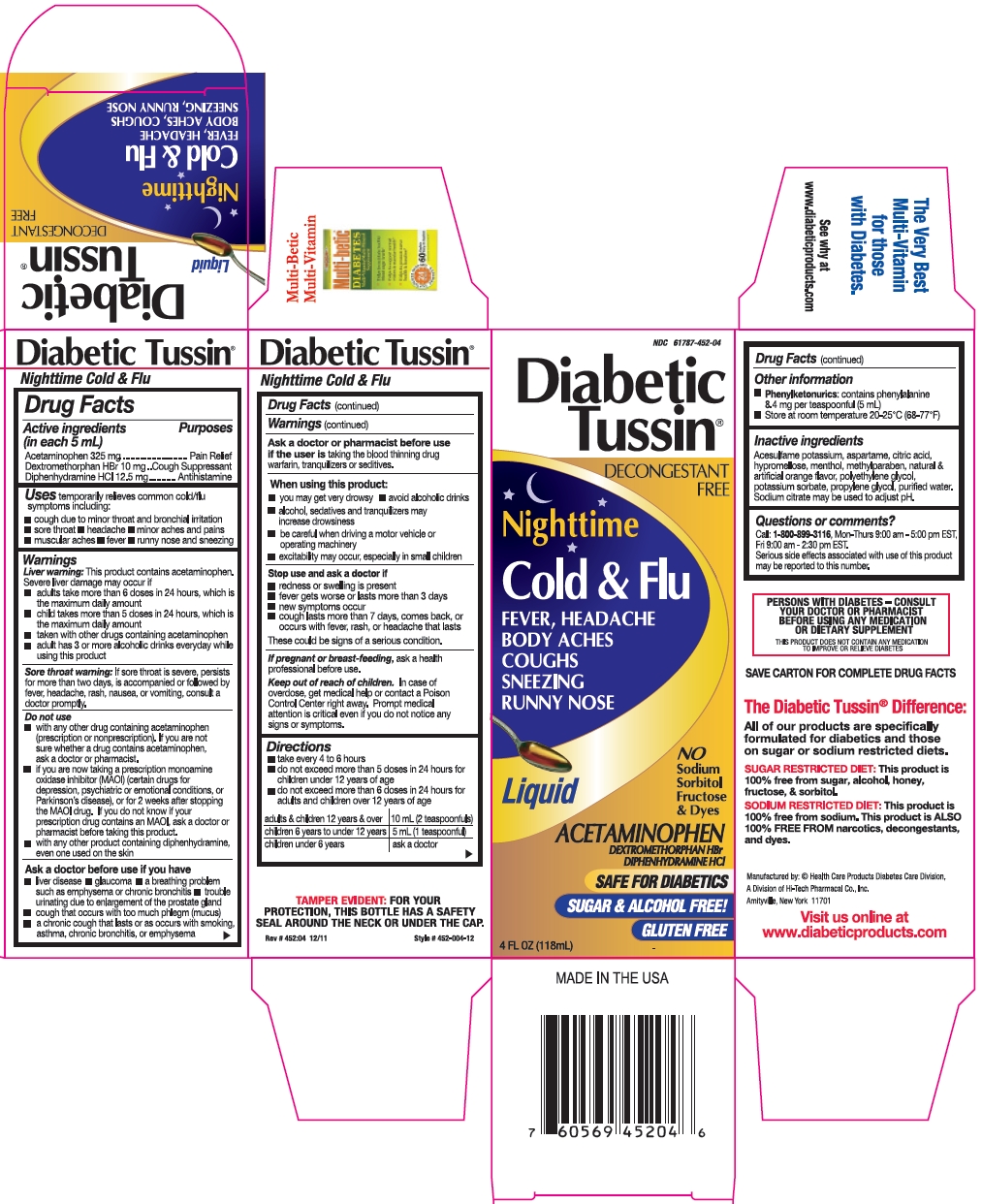
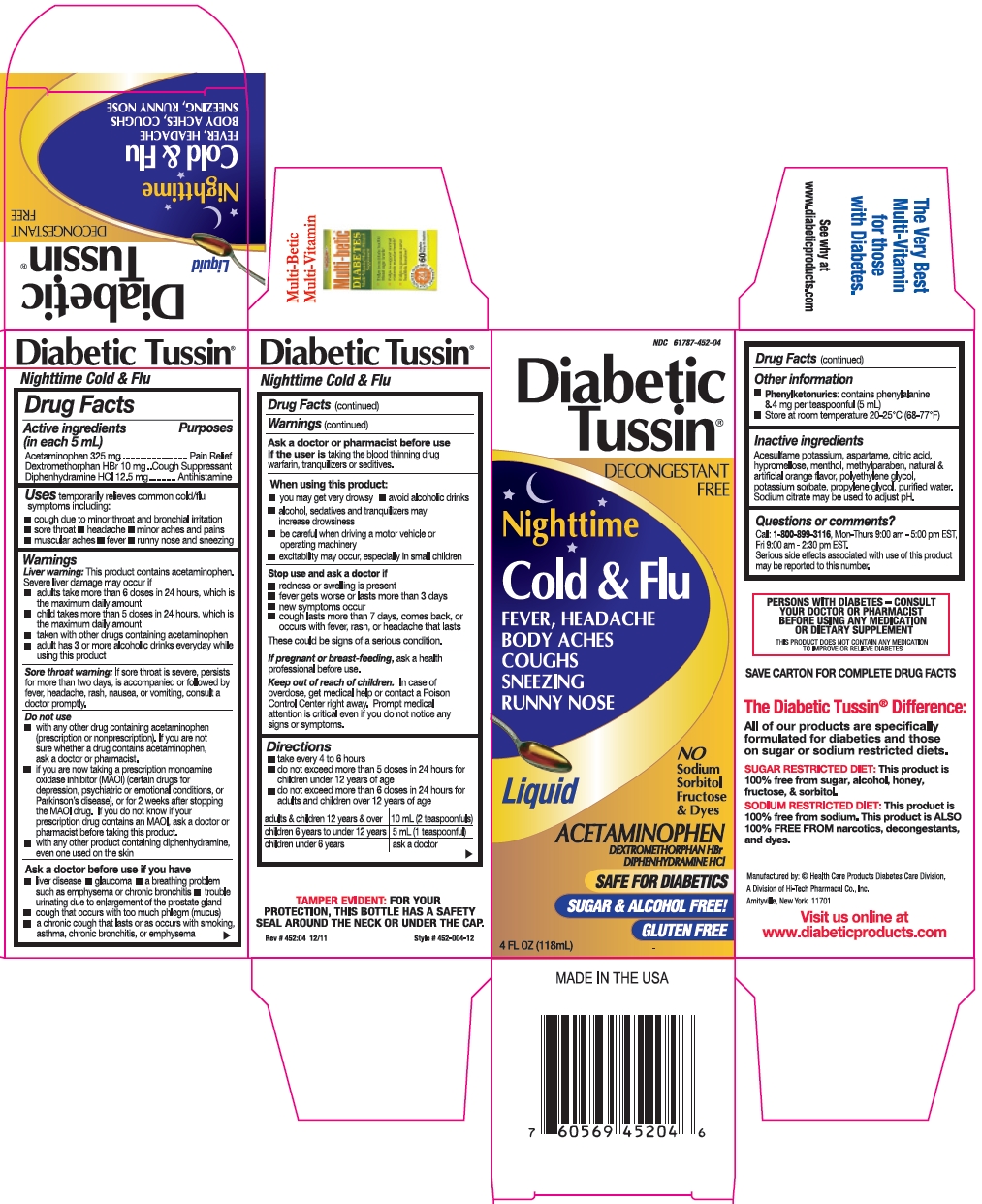
Label: DIABETIC TUSSIN NIGHTTIME COLD AND FLU- acetaminophen, dextromethorphan hbr, and diphenhydramine hcl liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 61787-452-04, 61787-452-08 - Packager: Health Care Products
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 17, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients (in each 5 mL)
- Purposes
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if
- •
- adults take more than 6 doses in 24 hours, which is the maximum daily amount
- •
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- •
- taken with other drugs containing acetaminophen
- •
- adult has 3 or more alcoholic drinks everyday while using this product
Sore throat warning: If sore throat is severe, persists for more than two days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- •
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- •
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- •
- with any other product containing diphenhydramine, even one used on the skin
Ask a doctor before use if you have
- •
- liver disease
- •
- glaucoma
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- trouble urinating due to enlargement of the prostate gland
- •
- cough that occurs with too much phlegm (mucus)
- •
- a chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis, or emphysema
Ask a doctor or pharmacist before use
if the user is taking the blood thinning drug warfarin, tranquilizers or sedatives.
When using this product:
- •
- you may get very drowsy
- •
- avoid alcoholic drinks
- •
- alcohol, sedatives and tranquilizers may increase drowsiness
- •
- be careful when driving a motor vehicle or operating machinery
- •
- excitability may occur, especially in small children
-
Directions
- •
- take every 4 to 6 hours
- •
- do not exceed more than 5 doses in 24 hours for children under 12 years of age
- •
- do not exceed more than 6 doses in 24 hours for adults and children over 12 years of age
adults & children 12 years & over
10 mL (2 teaspoonfuls)
children 6 years to under 12 years
5 mL (1 teaspoonful)
children under 6 years
ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DIABETIC TUSSIN NIGHTTIME COLD AND FLU
acetaminophen, dextromethorphan hbr, and diphenhydramine hcl liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61787-452 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg in 5 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 5 mL DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ASPARTAME (UNII: Z0H242BBR1) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) HYPROMELLOSE 2910 (4000 MPA.S) (UNII: RN3152OP35) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) METHYLPARABEN (UNII: A2I8C7HI9T) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) Product Characteristics Color BROWN (clear, colorless to light brown) Score Shape Size Flavor ORANGE (natural & artificial orange flavor) , MENTHOL Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61787-452-04 1 in 1 CARTON 08/01/2010 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:61787-452-08 1 in 1 CARTON 08/01/2010 2 237 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/01/2010 Labeler - Health Care Products (101196749) Registrant - Hi-Tech Pharmacal Co., Inc. (101196749) Establishment Name Address ID/FEI Business Operations Hi-Tech Pharmacal Co., Inc. 101196749 MANUFACTURE(61787-452)