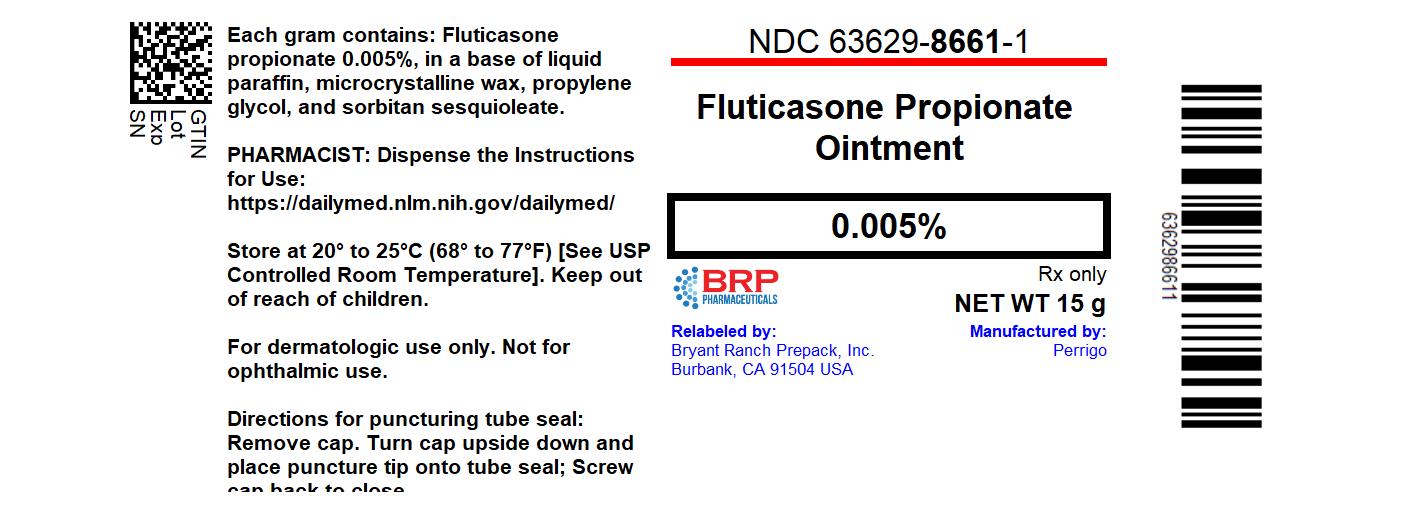
Label: FLUTICASONE PROPIONATE ointment
- NDC Code(s): 63629-8661-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 45802-221
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated April 4, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FLUTICASONE PROPIONATE OINTMENT safely and effectively. See full prescribing information for FLUTICASONE PROPIONATE OINTMENT.
FLUTICASONE PROPIONATE ointment, for topical use
Initial U.S. Approval: 1990RECENT MAJOR CHANGES
Warnings and Precautions, Ophthalmic Adverse Reaction (5.5) 03/2021
INDICATIONS AND USAGE
Fluticasone propionate ointment is a corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses in adult patients. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Ointment, 0.005%. (3)
CONTRAINDICATIONS
History of serious hypersensitivity to fluticasone propionate, or any other components of fluticasone propionate ointment. (4)
WARNINGS AND PRECAUTIONS
- •
- Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression: Reversible HPA axis suppression and resulting clinical glucocorticoid insufficiency can occur during or after withdrawal of treatment. Risk factors include use over large surface area, prolonged use, use under occlusion, altered skin barrier, liver failure, and young age. Modify use if HPA axis suppression is suspected. (5.1)
- •
- Local adverse reactions with topical corticosteroids may occur more frequently with the use of occlusive dressings, prolonged use, or use of higher potency corticosteroids. These reactions include: irritation, dryness, acneiform eruptions, hypertrichosis, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, striae and miliaria. (5.2)
- •
- Ophthalmic Adverse Reactions: May increase the risk of cataract and glaucoma. If visual symptoms occur, consider referral to an ophthalmologist. (5.5)
ADVERSE REACTIONS
The most common adverse reactions (<1%) were pruritus, burning, hypertrichosis, increased erythema, urticaria, irritation, and lightheadedness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Padagis® at 1-866-634-9120 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression and Other Adverse Endocrine Effects
5.2 Local Adverse Reactions
5.3 Allergic Contact Dermatitis
5.4 Skin Infections
5.5 Ophthalmic Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypothalamic-Pituitary-Adrenal (HPA) Axis Suppression and Other Adverse Endocrine Effects
Topical corticosteroids, including fluticasone propionate ointment, can produce reversible HPA axis suppression with the potential for clinical glucocorticoid insufficiency. Factors that predispose to HPA axis suppression include large treatment surface areas, prolonged use, use under occlusion, altered skin barrier, liver failure, and young age. Cushing’s syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids.
HPA axis suppression may occur during or after withdrawal of treatment. If HPA axis suppression is suspected, gradually withdraw the drug, reduce the frequency of application, or substitute with a less potent corticosteroid. Evaluation of HPA axis suppression may be done by using the cosyntropin stimulation test.
Pediatric patients may be at greater risk of HPA axis suppression due to their higher skin surface area to body mass ratios [see Use in Specific Populations (8.4)].
5.2 Local Adverse Reactions
The following local adverse reactions have been reported with topical corticosteroids. They may occur more frequently with the use of occlusive dressings and higher potency corticosteroids. These reactions are listed in an approximate decreasing order of occurrence: irritation, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, skin atrophy, striae, hypertrichosis, and miliaria.
5.3 Allergic Contact Dermatitis
Allergic contact dermatitis with topical corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation. Such an observation can be corroborated with appropriate diagnostic patch testing. Discontinue fluticasone propionate ointment if appropriate.
5.4 Skin Infections
If concomitant skin infections are present or develop, use an appropriate antimicrobial. If a favorable response does not occur promptly, discontinue use of fluticasone propionate ointment until the infection has been adequately controlled.
5.5 Ophthalmic Adverse Reactions
Use of topical corticosteroids may increase the risk of posterior subcapsular cataracts and glaucoma. Cataracts and glaucoma have been reported postmarketing with the use of topical corticosteroids.
Avoid contact of fluticasone propionate ointment with eyes. Advise patients to report any visual symptoms and consider referral to an ophthalmologist for evaluation.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- •
- HPA Axis Suppression and Other Adverse Endocrine Effects [see Warnings and Precautions (5.1)]
- •
- Local Adverse Reactions [see Warnings and Precautions (5.2)]
- •
- Concomitant Skin Infections [see Warnings and Precautions (5.4)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In controlled clinical trials, the total incidence of adverse reactions associated with the use of fluticasone propionate ointment was approximately 4%. These adverse reactions were usually mild, self-limiting, and consisted primarily of pruritus, burning, hypertrichosis, increased erythema, urticaria, irritation, and lightheadedness. Each of these events occurred individually in less than 1% of subjects.
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following local adverse reactions have been identified during post-approval use of fluticasone propionate ointment: acneiform dermatitis, edema, rash, hypoaesthesia, pustular psoriasis, skin atrophy.
The following systemic adverse reactions have been identified during post-approval use of fluticasone propionate ointment: immunosuppression/Pneumocystis jirovecii/pneumonia/leukopenia/thrombocytopenia; hyperglycemia/glycosuria; Cushing syndrome; generalized body edema/blurred vision; and acute urticarial reaction (edema, urticaria, pruritus, and throat swelling).
The following local adverse reactions have also been reported with the use of topical corticosteroids: telangiectasia, striae, dryness, folliculitis, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria.
Ophthalmic adverse reactions including cataracts, glaucoma and increased intraocular pressure have been reported with the use of topical corticosteroids.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on fluticasone propionate ointment use in pregnant women to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. Observational studies suggest an increased risk of low birthweight infants with the use of greater than 300 grams of potent or very potent topical corticosteroids during pregnancy (see Data). Advise pregnant women that fluticasone propionate ointment may increase the risk of having a low birthweight infant and to use fluticasone propionate ointment on the smallest area of skin and for the shortest duration possible.
In animal reproduction studies, subcutaneous administration of fluticasone propionate to pregnant mice, rats, and rabbits during organogenesis caused malformations characteristic of corticosteroids in each species (see Data). The available data do not allow the calculation of relevant comparisons between the systemic exposure of fluticasone propionate observed in animal studies to the systemic exposure that would be expected in humans after topical use of fluticasone propionate ointment.
The background risk of major birth defects and miscarriage for the indicated population(s) is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
Available observational studies in pregnant women did not identify a drug-associated risk of major birth defects, preterm delivery, or fetal mortality with the use of topical corticosteroids of any potency. However, when the dispensed amount of potent or very potent topical corticosteroids exceeded 300 grams during the entire pregnancy, maternal use was associated with an increased risk of low birth weight infants.
Animal Data
In embryo-fetal development studies, pregnant rabbits, rats, and mice received subcutaneous doses of fluticasone propionate during organogenesis at doses up to 4, 100, and 150 μg/kg/day, respectively. A malformation characteristic of corticosteroids (cleft palate) was noted at the high dose in each species. Additional adverse effects were noted in rats and rabbits. Decreased fetal weights and retarded skeletal ossification were noted in rabbits at 4 μg/kg/day and rats at 100 μg/kg/day. Maternal toxicity and omphalocele were also noted in rats at 100 μg/kg/day. No malformations or developmental toxicity was noted in rabbits at 0.57 μg/kg/day, in rats at 10 μg/kg/day, or in mice at 15 μg/kg/day. Fluticasone propionate crossed the placenta following administration of a subcutaneous or an oral dose of 100 μg/kg tritiated fluticasone propionate to pregnant rats.
8.2 Lactation
Risk Summary
There are no data on the presence of fluticasone propionate in human milk, its effects on the breastfed infant, or its effects on milk production. It is not known whether topical administration of fluticasone propionate ointment could result in sufficient systemic absorption to produce detectable quantities in human milk (see Clinical Considerations).
The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for fluticasone propionate ointment and any potential adverse effects on the breastfed child from fluticasone propionate ointment or from the underlying maternal condition.
Clinical Considerations
To minimize potential exposure to the breastfed infant via breast milk, use fluticasone propionate ointment on the smallest area of skin and for the shortest duration possible while breastfeeding. Advise breastfeeding women not to apply fluticasone propionate ointment directly to the nipple and areola prior to breastfeeding to avoid direct infant exposure [see Use in Specific Populations (8.4)].
8.4 Pediatric Use
The safety and effectiveness of fluticasone propionate ointment have not been established in pediatric patients. Use of fluticasone propionate ointment in pediatric patients is not recommended.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of systemic effects when treated with topical drugs. They are, therefore, also at greater risk of HPA axis suppression and adrenal insufficiency upon the use of topical corticosteroids [see Warnings and Precautions (5.1)].
In a trial of 35 pediatric subjects treated with fluticasone propionate ointment, 0.005% for atopic dermatitis over at least 35% of body surface area, subnormal adrenal function was observed with cosyntropin stimulation testing at the end of 3 to 4 weeks of treatment in 4 subjects who had normal testing prior to treatment. It is not known if these subjects had recovery of adrenal function because follow-up testing was not performed. The decreased responsiveness of cosyntropin testing was not correlated to age of subject, amount of fluticasone propionate ointment used, or serum levels of fluticasone propionate.
In the above trial, telangiectasia on the face was noted in one subject on the eighth day of a 4-week treatment period. Facial use was discontinued and the telangiectasia resolved.
HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids.
8.5 Geriatric Use
A limited number of patients above 65 years of age (n=203) have been treated with fluticasone propionate ointment in US and non-US clinical trials. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. However, greater sensitivity of some older individuals cannot be ruled out.
-
11 DESCRIPTION
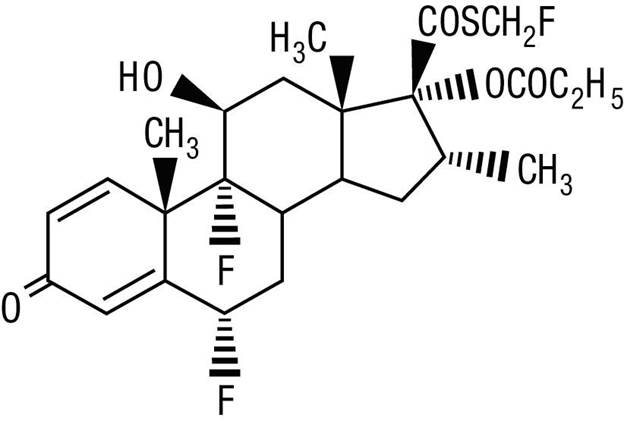
Fluticasone Propionate Ointment, 0.005% contains fluticasone propionate [S-Fluoromethyl 6α, 9α-difluoro-11β-hydroxy-16α-methyl-3-oxo-17α-propionyloxyandrosta-1,4-diene-17β-carbothioate], a synthetic fluorinated corticosteroid, for topical use.
Chemically, fluticasone propionate is C25H31F3O5S. It has the following structural formula:
Fluticasone propionate has a molecular weight of 500.6. It is a white to off-white powder and is insoluble in water.
Each gram of fluticasone propionate ointment contains fluticasone propionate 0.05 mg in a white to off-white translucent ointment base of liquid paraffin, microcrystalline wax, propylene glycol, and sorbitan sesquioleate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Corticosteroids play a role in cellular signaling, immune function, inflammation, and protein regulation; however, the precise mechanism of action of fluticasone propionate ointment in corticosteroid-responsive dermatoses is unknown.
12.2 Pharmacodynamics
Vasoconstrictor Assay
Studies performed with fluticasone propionate ointment indicate that it is in the medium range of potency as demonstrated in vasoconstrictor trials in healthy subjects when compared with other topical corticosteroids. However, similar blanching scores do not necessarily imply therapeutic equivalence.
12.3 Pharmacokinetics
Absorption
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressing enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption.
In a study of 6 healthy subjects applying 25 g of fluticasone propionate ointment 0.005% twice daily to the trunk and legs for up to 5 days under occlusion, plasma levels of fluticasone ranged from 0.08 to 0.22 ng/mL.
Distribution
The percentage of fluticasone propionate bound to human plasma proteins averaged 91%. Fluticasone propionate is weakly and reversibly bound to erythrocytes. Fluticasone propionate is not significantly bound to human transcortin.
Metabolism
No metabolites of fluticasone propionate were detected in an in vitro study of radiolabeled fluticasone propionate incubated in human skin homogenate.
Fluticasone propionate is metabolized in the liver by cytochrome P450 3A4-mediated hydrolysis of the 5-fluoromethyl carbothioate grouping. This transformation occurs in 1 metabolic step to produce the inactive 17-β-carboxylic acid metabolite, the only known metabolite detected in man. This metabolite has approximately 2,000 times less affinity than the parent drug for the glucocorticoid receptor of human lung cytosol in vitro and negligible pharmacological activity in animal studies. Other metabolites detected in vitro using cultured human hepatoma cells have not been detected in man.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In an oral (gavage) mouse carcinogenicity study, doses of 0.1, 0.3, and 1 mg/kg/day fluticasone propionate were administered to mice for 18 months. Fluticasone propionate demonstrated no tumorigenic potential at oral doses up to 1 mg/kg/day in this study.
In a dermal mouse carcinogenicity study, 0.05% fluticasone propionate ointment (40 μl) was topically administered for 1, 3 or 7 days/week for 80 weeks. Fluticasone propionate demonstrated no tumorigenic potential at dermal doses up to 6.7 μg/kg/day in this study.
Fluticasone propionate was not mutagenic or clastogenic in five in vitro genotoxicity tests (Ames assay, E. coli fluctuation test, S. cerevisiae gene conversion test, Chinese hamster ovary cell chromosome aberration assay and human lymphocyte chromosome aberration assay) and one in vivo genotoxicity test (mouse micronucleus assay).
In a fertility study, male and female rats received subcutaneous doses of fluticasone propionate at doses up to 50 μg/kg/day. No impairment of fertility or mating performance was observed.
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advise the patient:
- •
- Avoid contact with the eyes.
- •
- Do not bandage the treated skin area, or cover or wrap it to cause occlusion unless directed by the healthcare provider.
- •
- Report any signs of local adverse reaction to their healthcare provider.
- •
- Do not use on the face, underarms, or groin areas unless directed by the healthcare provider.
- •
- Advise a woman to use fluticasone propionate ointment on the smallest area of skin and for the shortest duration possible while pregnant or breastfeeding. Advise breastfeeding women not to apply fluticasone propionate ointment directly to the nipple and areola to avoid direct infant exposure.
Manufactured by Padagis®
Yeruham, Israel
www.padagis.comRev 11-23
57B00 RC PH2 -
PATIENT INFORMATION
Fluticasone Propionate (floo-TIK-a-sone PROE-pee-oh-nate) Ointment, 0.005%
Important: Fluticasone propionate ointment is for use on skin only (topical).
Do not get fluticasone propionate ointment near or in your eyes, mouth, or vagina.
Read this Patient Information before you start using fluticasone propionate ointment and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is fluticasone propionate ointment?
Fluticasone propionate ointment is a prescription corticosteroid medicine used on the skin (topical) for the relief of inflammation and itching caused by certain skin conditions in adults.
It is not known if fluticasone propionate ointment is safe and effective in children. Fluticasone propionate ointment is not recommended for use in children.
Before using fluticasone propionate ointment, tell your healthcare provider about all of your medical conditions, including if you:
- •
- have an allergy to any of the ingredients in fluticasone propionate ointment
- •
- have a skin infection at the site to be treated.
You may also need medicine to treat the skin infection.
- •
- have adrenal gland problems
- •
- have liver problems
- •
- have diabetes
- •
- have thinning skin (atrophy) at the site to be treated
- •
- have cataracts or glaucoma
- •
- are pregnant or plan to become pregnant. It is not known if fluticasone propionate ointment will harm your unborn baby. If you use fluticasone propionate ointment during pregnancy, use fluticasone propionate ointment on the smallest area of the skin and for the shortest time needed.
- •
- are breastfeeding or plan to breastfeed. It is not known if fluticasone propionate ointment can pass into your breast milk and harm your baby. Breastfeeding women should use fluticasone propionate ointment on the smallest area of skin and for the shortest time needed. Do not apply fluticasone propionate ointment directly to the nipple and areola to avoid contact with your baby.
Tell your healthcare provider about all the medicines you take,
including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take other corticosteroid medicines by mouth or use other products on your skin that contain corticosteroids.
How should I use fluticasone propionate ointment?
- •
- Use fluticasone propionate ointment exactly as your healthcare provider tells you to use it.
- •
- Apply a thin film of fluticasone propionate ointment to the affected area 2 times each day. Gently rub into your skin.
- •
- Do not bandage, cover, or wrap the treated area unless your healthcare provider tells you to.
- •
- Do not use fluticasone propionate ointment on your face, groin, underarms (armpits), unless your healthcare provider tells you to.
- •
- Wash your hands after applying fluticasone propionate ointment, unless your hands are being treated.
- •
- Tell your healthcare provider if your symptoms get worse with fluticasone propionate ointment or if your symptoms do not improve after 2 weeks of treatment.
What are possible side effects of fluticasone propionate ointment?
Fluticasone propionate ointment may cause serious side effects, including:
- •
- Fluticasone propionate ointment can pass through your skin and may cause adrenal gland problems. This is more likely to happen if you use fluticasone propionate ointment for too long, use it over a large treatment area, use it with other topical medicines that contain corticosteroids, cover the treated area, or have liver failure. Your healthcare provider may do blood tests to check your adrenal gland function during and after treatment with fluticasone propionate ointment.
- •
- Skin problems, including skin reactions or thinning of your skin (atrophy), skin infections, and allergic reactions (allergic contact dermatitis) at the treatment site. Tell your healthcare provider if you get any skin reactions such as pain, tenderness, swelling, or healing problems.
- •
- Vision Problems. Topical corticosteroids including fluticasone propionate ointment may increase your chance of developing cataract(s) and glaucoma. Tell your healthcare provider if you develop blurred vision or other vision problems during treatment with fluticasone propionate ointment.
The most common side effects of fluticasone propionate ointment include itching, burning, excessive hair growth, skin redness, hives, and lightheadedness.
These are not all the possible side effects of fluticasone propionate ointment. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store fluticasone propionate ointment?
- •
- Store fluticasone propionate ointment between 20-25°C (68-77°F).
Keep fluticasone propionate ointment and all medicines out of the reach of children.
General information about the safe and effective use of fluticasone propionate ointment.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use fluticasone propionate ointment for a condition for which it was not prescribed. Do not give fluticasone propionate ointment to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about fluticasone propionate ointment that is written for health professionals.
What are the ingredients in fluticasone propionate ointment?
Active ingredient: fluticasone propionate
Inactive ingredients: liquid paraffin, microcrystalline wax, propylene glycol, and sorbitan sesquioleate
This Patient Information has been approved by the U.S. Food and Drug Administration.
Manufactured by Padagis®
Yeruham, Israel
www.padagis.comRev 11-23
57B00 RC PH2
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FLUTICASONE PROPIONATE
fluticasone propionate ointmentProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:63629-8661(NDC:45802-221) Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLUTICASONE PROPIONATE (UNII: O2GMZ0LF5W) (FLUTICASONE - UNII:CUT2W21N7U) FLUTICASONE PROPIONATE 0.05 mg in 1 g Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63629-8661-1 1 in 1 CARTON 02/12/2008 1 15 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076668 07/09/2007 Labeler - Bryant Ranch Prepack (171714327) Registrant - Bryant Ranch Prepack (171714327) Establishment Name Address ID/FEI Business Operations Bryant Ranch Prepack 171714327 REPACK(63629-8661) , RELABEL(63629-8661)