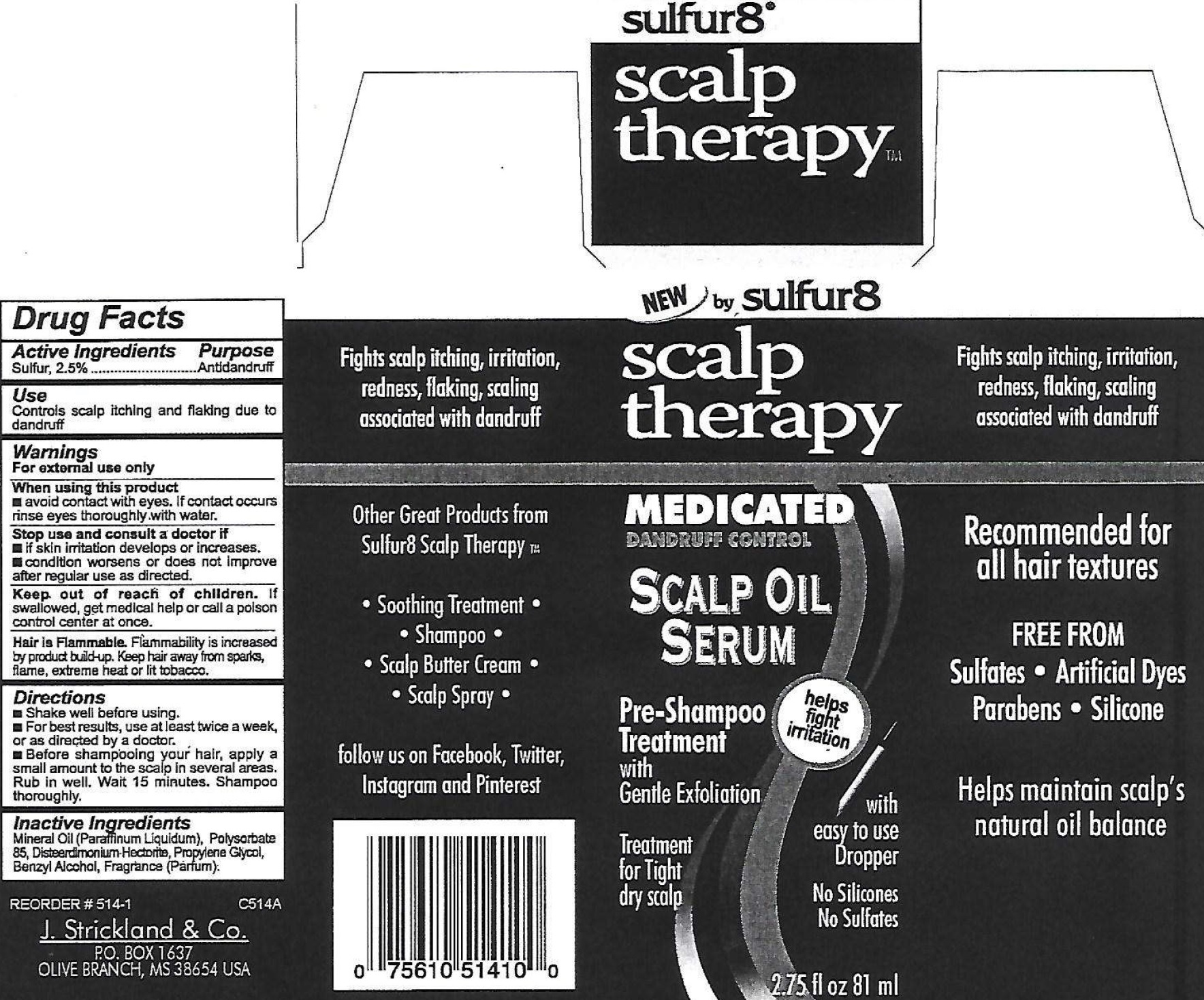
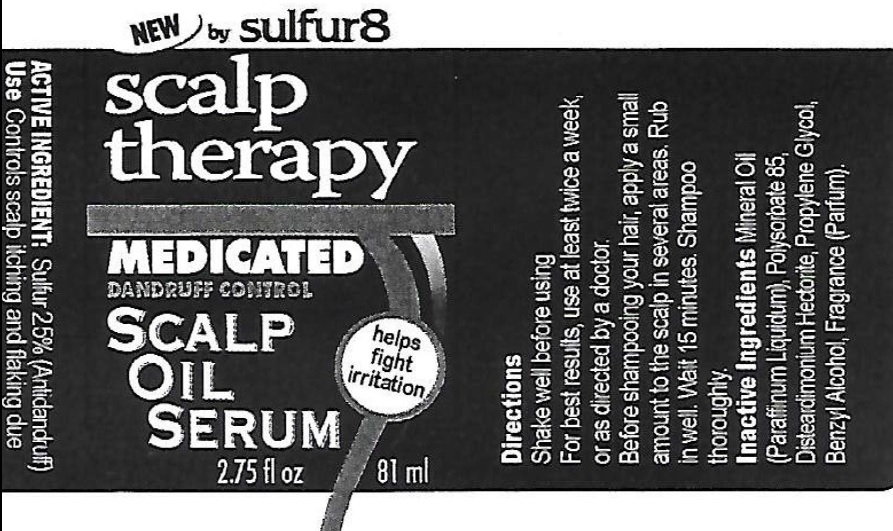
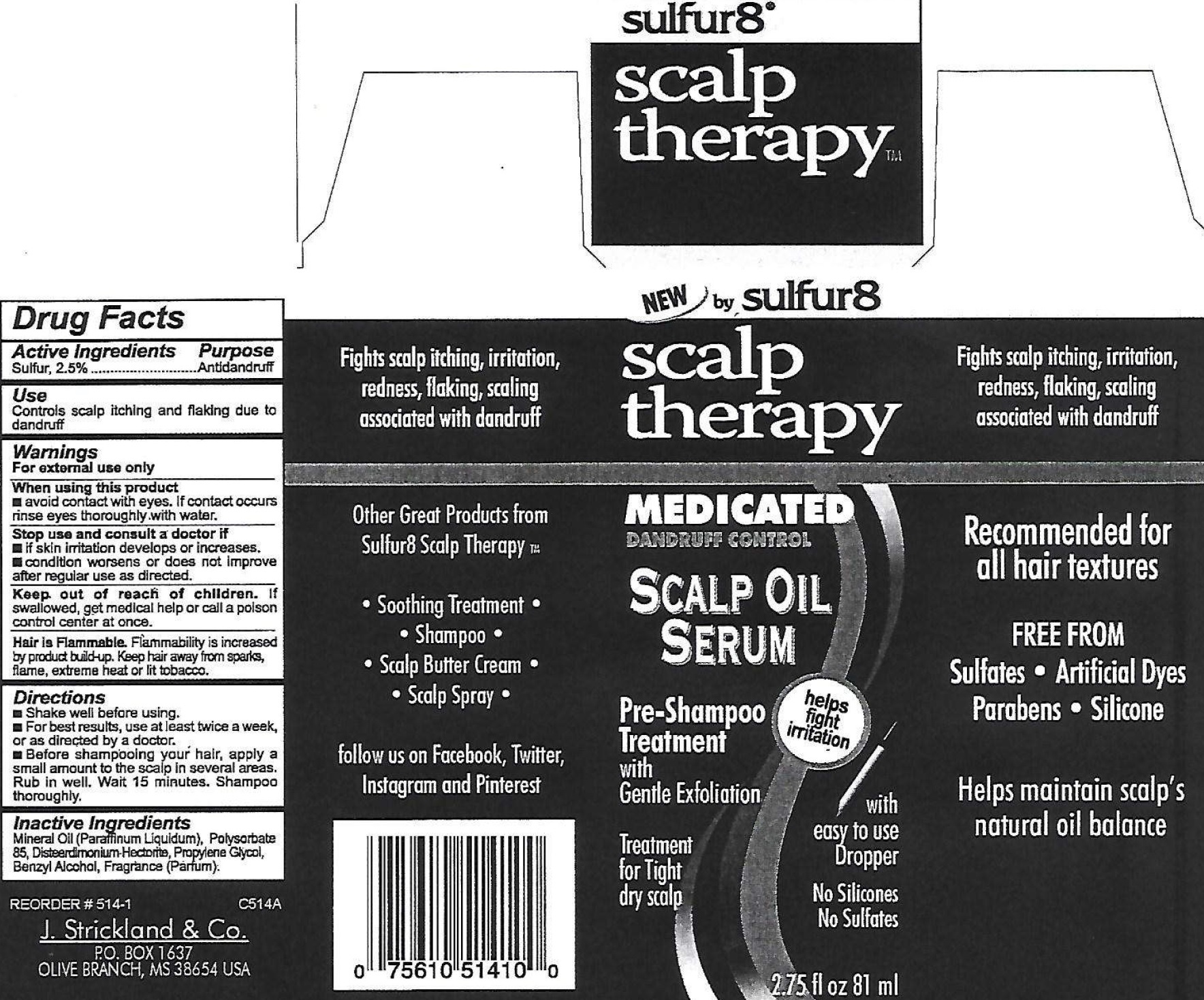
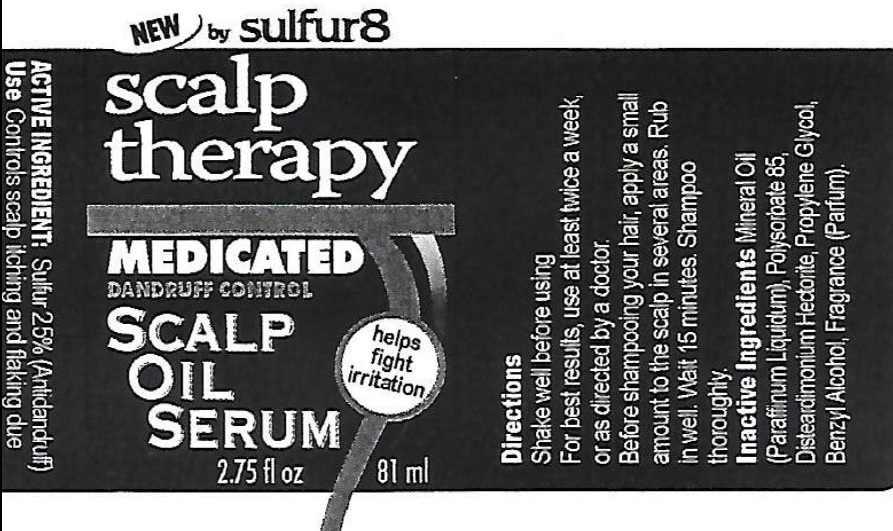
Label: SULFUR 8 SCALP THERAPY MEDICATED DANDRUFF CONTROL SCALP OIL SERUM- sulfur suspension
- NDC Code(s): 12022-034-00
- Packager: J. Strickland and Co.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated October 21, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Use
- Warnings
- Directions
- Inactive Ingredients
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
SULFUR 8 SCALP THERAPY MEDICATED DANDRUFF CONTROL SCALP OIL SERUM
sulfur suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:12022-034 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 25 mg in 1 mL Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) POLYSORBATE 85 (UNII: A7F3N56197) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:12022-034-00 1 in 1 BOX 01/01/2020 1 81 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 01/01/2020 Labeler - J. Strickland and Co. (007023112)