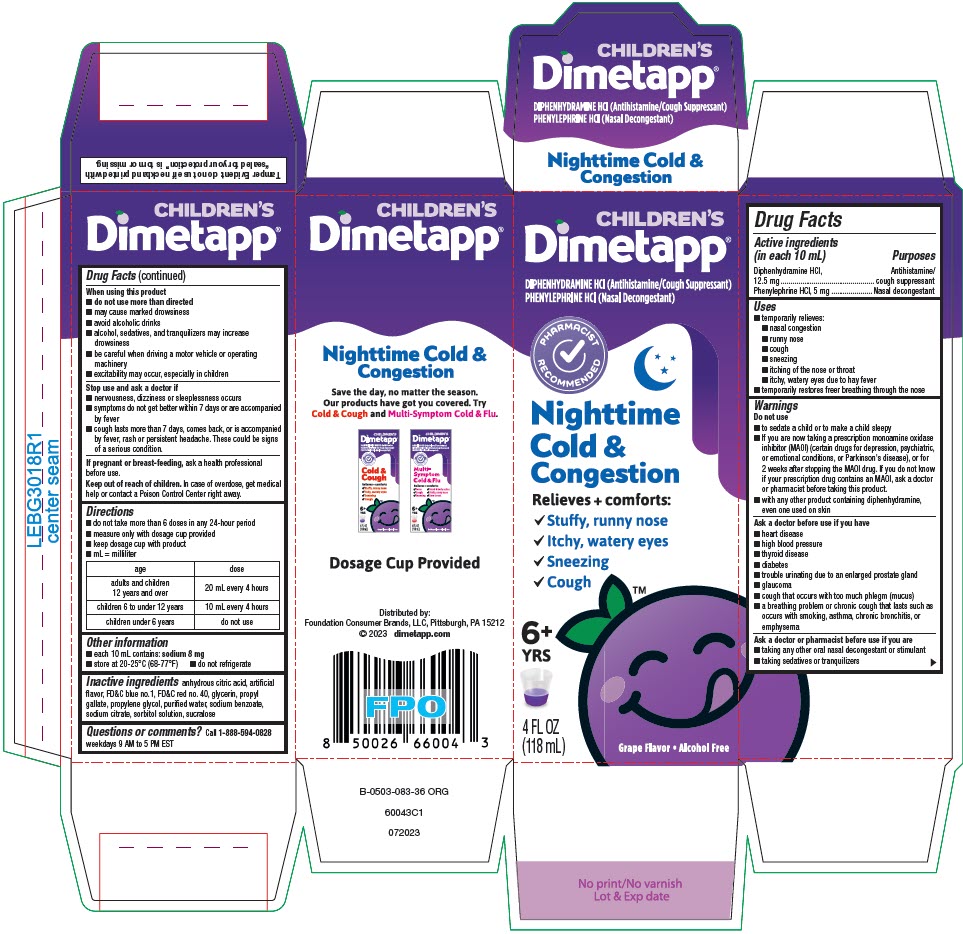
Label: DIMETAPP NIGHTTIME COLD AND CONGESTION- diphenhydramine hydrochloride and phenylephrine hydrochloride solution
- NDC Code(s): 80070-340-04
- Packager: Foundation Consumer Brands
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 3, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
-
Warnings
Do not use
- to sedate a child or to make a child sleepy
- If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- glaucoma
- cough that occurs with too much phlegm (mucus)
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis, or emphysema
Ask a doctor or pharmacist before use if you are
- taking any other oral nasal decongestant or stimulant
- taking sedatives or tranquilizers
When using this product
- do not use more than directed
- may cause marked drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
-
INGREDIENTS AND APPEARANCE
DIMETAPP NIGHTTIME COLD AND CONGESTION
diphenhydramine hydrochloride and phenylephrine hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80070-340 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 6.25 mg in 5 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 2.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) Product Characteristics Color PURPLE Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80070-340-04 1 in 1 CARTON 09/15/2021 1 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M012 09/15/2021 Labeler - Foundation Consumer Brands (117603632)