Label: NITROGEN- nitrogen gas
- NDC Code(s): 52003-016-20
- Packager: Messer Canada Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved medical gas
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- WARNINGS AND PRECAUTIONS SECTION
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Messer
Gases for Life
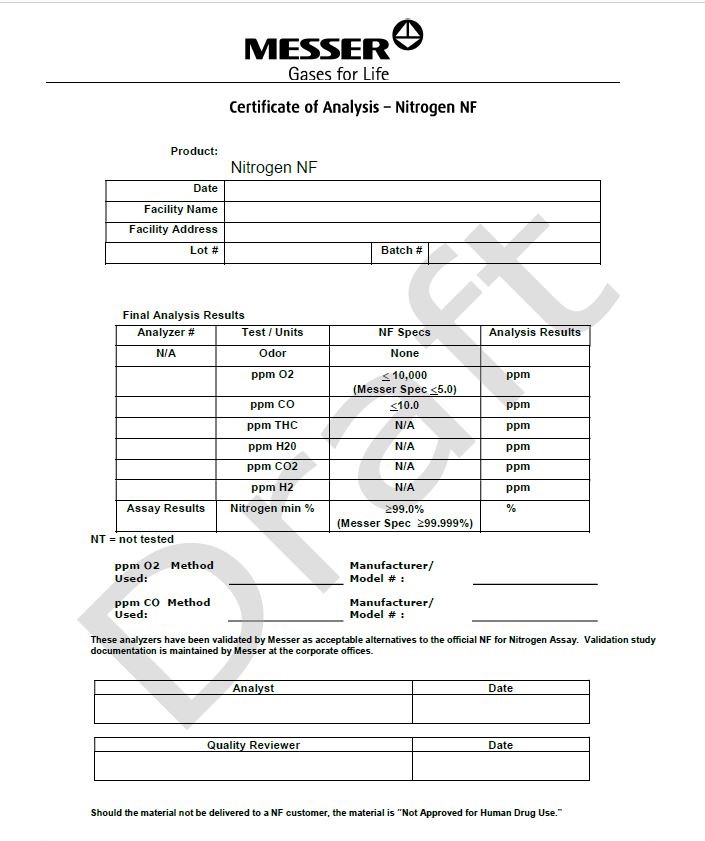
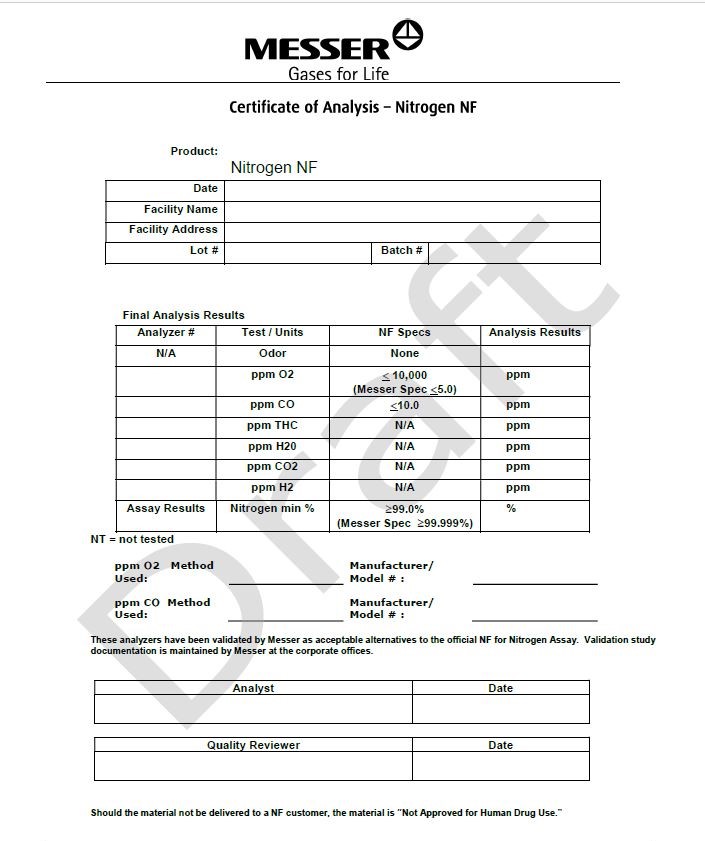
Certicate of Analysis - Nitrogen NF
Product: Nitrogen NF
Date
Facility Name
Facility Address
Lot#
Batch #
Final Analysis Results
Analyzer #
Test/Units
NF Specs
Analysis Results
N/A
Odor
None
ppm O2
< 10,000
(Messer Spec <5.0)
ppm
ppm CO
< 10.0
ppm
ppm THC
N/A
ppm
ppm H2O
N/A
ppm
ppm CO2
N/A
ppm
ppm H2
N/A
ppm
Assay Results
Nitrogen min %
> 99.0%
(Messer Spec >99.999%)
%
NT= not tested
PPM O2 Method Used: __________________________
Manufacturer/Model #: __________________________
PPM O2 Method Used: __________________________
Manufacturer/Model #: __________________________
This analyzer has been validated by Messer as acceptable alternatives to the official NF for Nitrogen Assay. Validation study documentation is maintained by Messer at the corporate offices.
Analyst
Date
Quality Reviewer
Date
Should the material not be delivered to a NF customer, the material is “Not Approved for Human Drug Use.”
-
INGREDIENTS AND APPEARANCE
NITROGEN
nitrogen gasProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:52003-016 Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Nitrogen (UNII: N762921K75) (Nitrogen - UNII:N762921K75) Nitrogen 995 mL in 1 L Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52003-016-20 25000 L in 1 TANK; Type 0: Not a Combination Product 01/01/1960 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved medical gas 01/01/1960 Labeler - Messer Canada Inc. (201613189) Registrant - Messer Canada Inc. (201613189) Establishment Name Address ID/FEI Business Operations Messer Canada Inc. 244231937 manufacture(52003-016) , api manufacture(52003-016)