Label: CRANE SAFETY BISMUTH- bismuth subsalicylate tablet, chewable
- NDC Code(s): 73408-406-20
- Packager: Crane Safety LLC
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 23, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
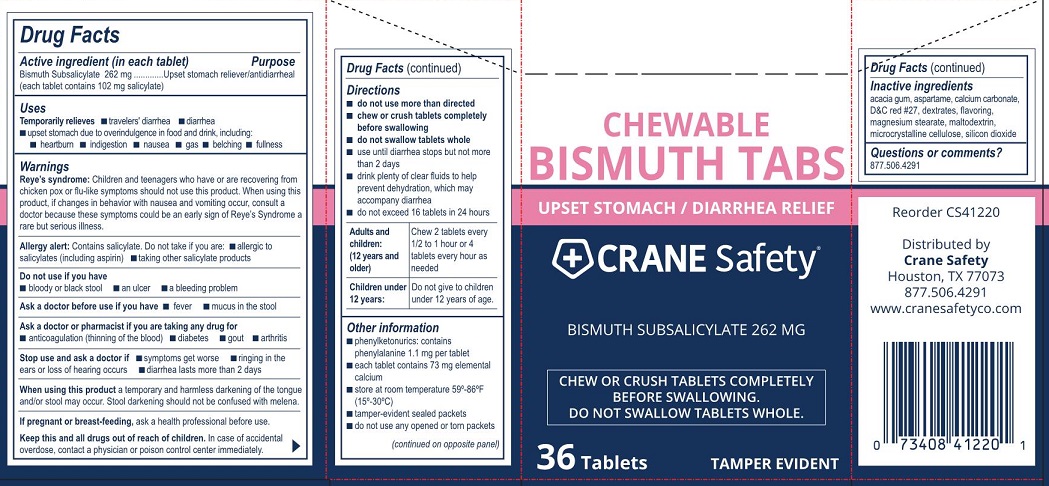
- Drug Facts
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's Syndrome a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are:
- allergic to salicylates (including aspirin)
- taking other salicylate products
- Do not use if you have
- Ask a doctor before use if you have
- Ask a doctor or pharmacist if you are taking any drug for
- Stop use and ask a doctor if
- When using this product
- If pregnant or breast-feeding,
- Keep this and all drugs out of reach of children.
-
Directions
- do not use more than directed
- chew or crush tablets completely befre swallowing
- do not swallow tablet whole
- use until diarrhea stops but not more than 2 days
- drink plenty of clear fluids to help prevent dehydration, which may accompany diarrhea
- do not exceed 16 tablets in 24 hours
Adults and children: (12 years and older) Chew 2 tablets every 1/2 to 1 hour or 4 tablets every hour as needed
Children under 12 years: Do not give to children under 12 years of age.
- Other information
- Inactive ingredients
- Questions or comments?
- Crane Safety Bismuth Label
-
INGREDIENTS AND APPEARANCE
CRANE SAFETY BISMUTH
bismuth subsalicylate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73408-406 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BISMUTH SUBSALICYLATE (UNII: 62TEY51RR1) (SALICYLIC ACID - UNII:O414PZ4LPZ) BISMUTH SUBSALICYLATE 262 mg Inactive Ingredients Ingredient Name Strength D&C RED NO. 27 (UNII: 2LRS185U6K) CALCIUM CARBONATE (UNII: H0G9379FGK) DEXTRATES (UNII: G263MI44RU) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ACACIA (UNII: 5C5403N26O) ASPARTAME (UNII: Z0H242BBR1) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MALTODEXTRIN (UNII: 7CVR7L4A2D) Product Characteristics Color pink Score no score Shape ROUND Size 16mm Flavor Imprint Code RH;046 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73408-406-20 6 in 1 BOX 01/17/2020 11/29/2024 1 6 in 1 CELLO PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part335 01/17/2020 11/29/2024 Labeler - Crane Safety LLC (080998015) Registrant - Unifirst First Aid Corporation (832947092)