Label: DRAMAMINE- dimenhydrinate tablet, chewable
DRAMAMINE, TRAVEL BASIX- dimenhydrinate tablet, chewable
- NDC Code(s): 66715-6409-2, 66715-9809-1, 66715-9809-2
- Packager: Lil' Drug Store Products, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
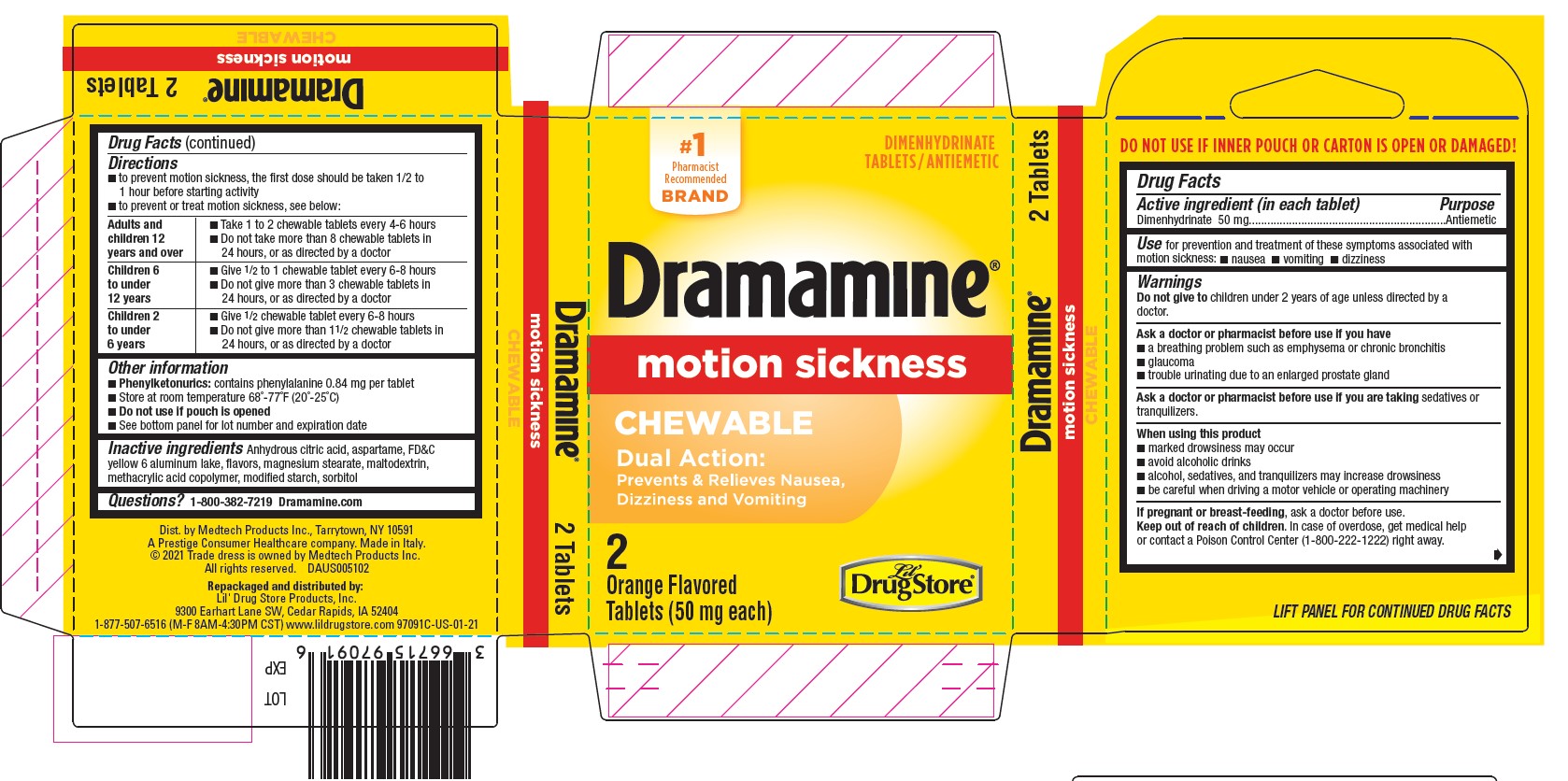
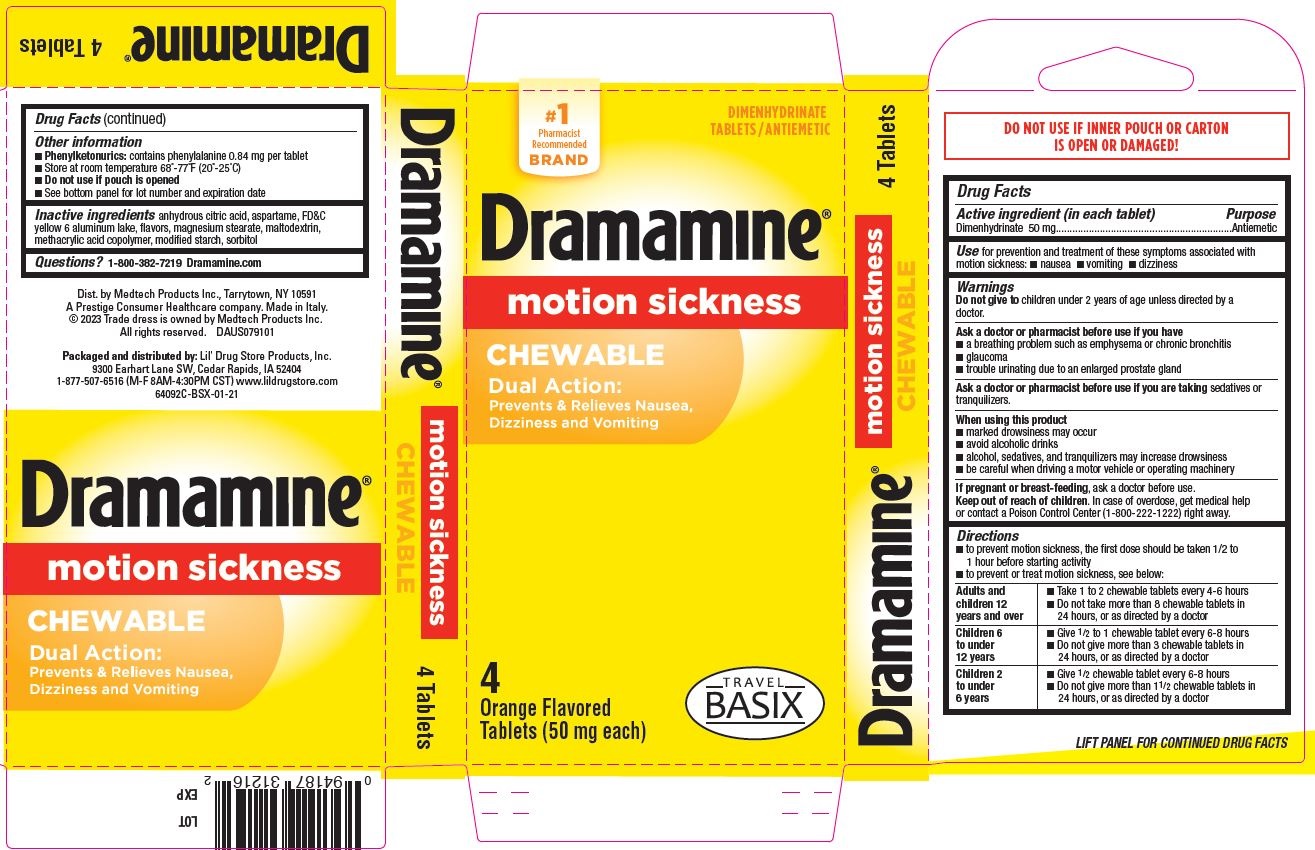
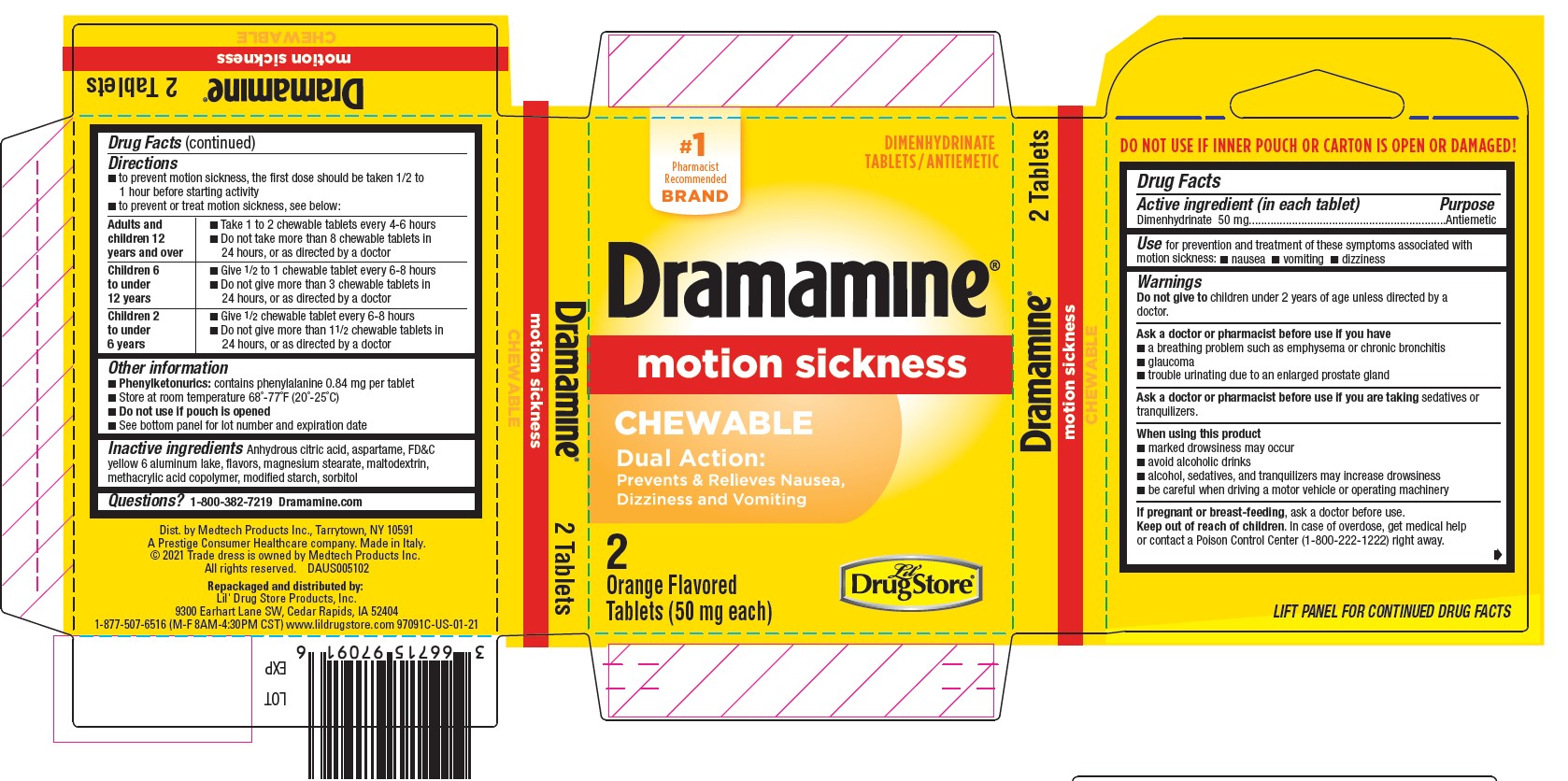
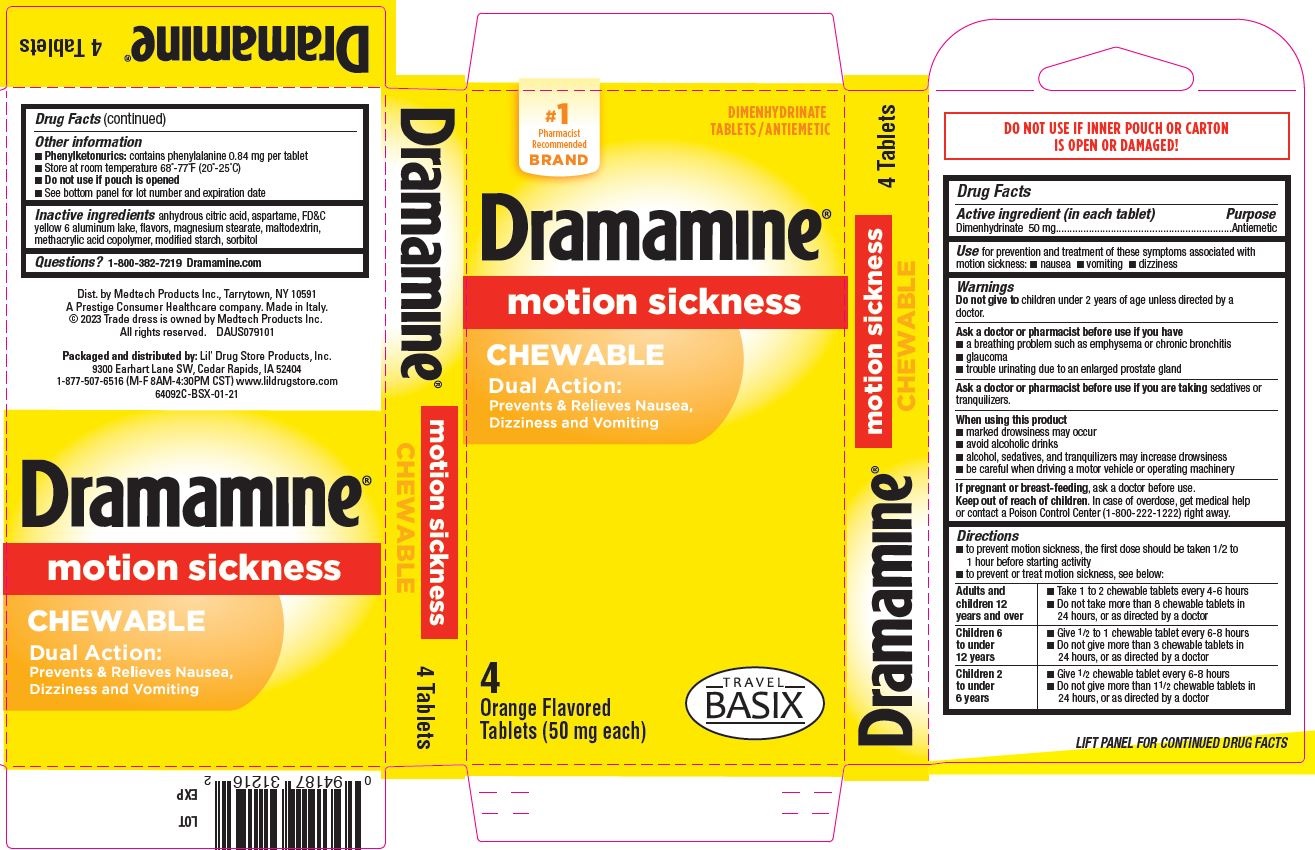
- Active ingredient (in each tablet)
- Purpose
- Use
-
Warnings
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- trouble urinating due to an enlarged prostate gland
-
Directions
- to prevent motion sickness, the first dose should be taken 1/2 to 1 hour before starting activity
- to prevent or treat motion sickness, see below:
adults and children 12 years and over - take 1 to 2 chewable tablets every 4-6 hours
- do not take more than 8 chewable tablets in 24 hours, or as directed by a doctor
children 6 to under 12 years - give ½ to 1 chewable tablet every 6-8 hours
- do not give more than 3 chewable tablets in 24 hours, or as directed by a doctor
children 2 to under 6 years - give ½ chewable tablet every 6-8 hours
- do not give more than 1-½ chewable tablets in 24 hours, or as directed by a doctor
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- Dramamine Chewable 2ct, Lil' Drug Store - PDP/Package
- Dramamine Chewable 4ct, TRAVEL BASIX - PDP/Package
-
INGREDIENTS AND APPEARANCE
DRAMAMINE
dimenhydrinate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66715-9809 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMENHYDRINATE (UNII: JB937PER5C) (CHLORTHEOPHYLLINE - UNII:GE2UA340FM) DIMENHYDRINATE 50 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) ALUMINUM OXIDE (UNII: LMI26O6933) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) STARCH, CORN (UNII: O8232NY3SJ) SORBITOL (UNII: 506T60A25R) Product Characteristics Color orange Score 2 pieces Shape ROUND Size 12mm Flavor ORANGE Imprint Code DRA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66715-9809-1 1 in 1 CARTON 08/26/2016 1 2 in 1 POUCH; Type 0: Not a Combination Product 2 NDC:66715-9809-2 2 in 1 CARTON 05/16/2022 2 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 05/10/2010 DRAMAMINE, TRAVEL BASIX
dimenhydrinate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66715-6409 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMENHYDRINATE (UNII: JB937PER5C) (CHLORTHEOPHYLLINE - UNII:GE2UA340FM) DIMENHYDRINATE 50 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ASPARTAME (UNII: Z0H242BBR1) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) ALUMINUM OXIDE (UNII: LMI26O6933) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) METHACRYLIC ACID - METHYL METHACRYLATE COPOLYMER (1:1) (UNII: 74G4R6TH13) STARCH, CORN (UNII: O8232NY3SJ) SORBITOL (UNII: 506T60A25R) Product Characteristics Color orange Score 2 pieces Shape ROUND Size 12mm Flavor ORANGE Imprint Code DRA Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66715-6409-2 1 in 1 CARTON 11/28/2022 1 2 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M009 11/28/2022 Labeler - Lil' Drug Store Products, Inc. (093103646)