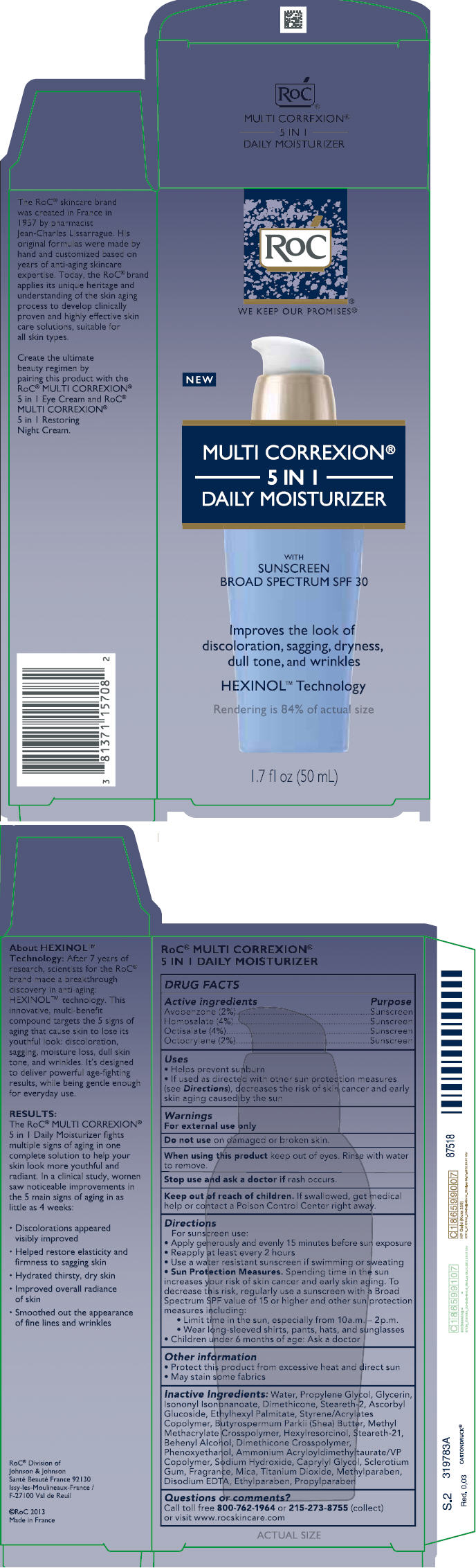
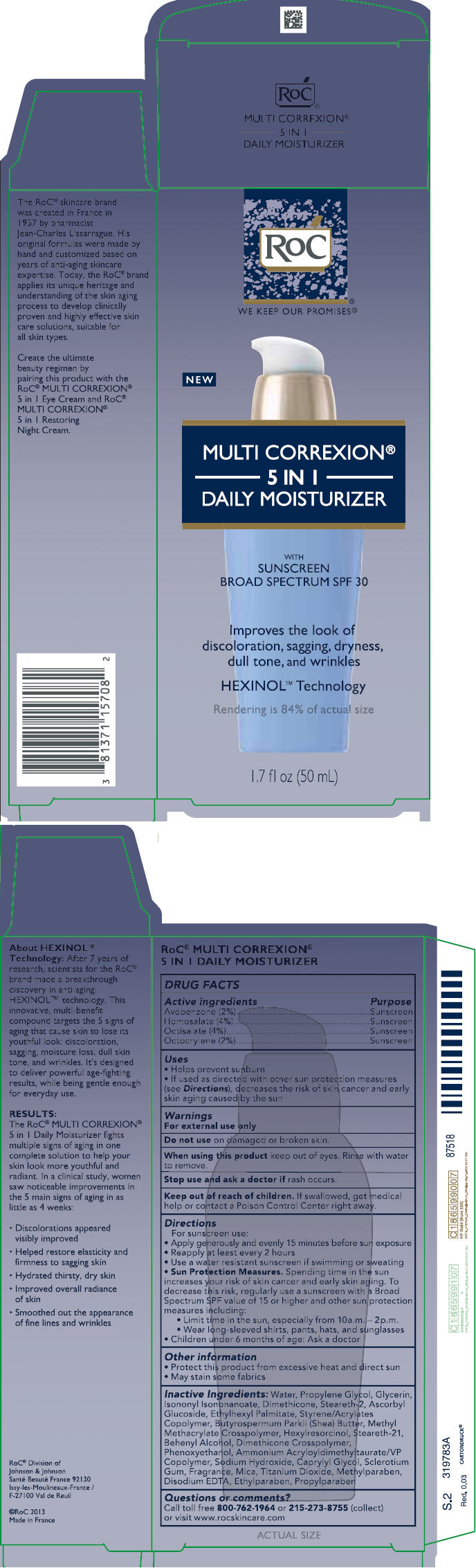
Label: ROC MULTI CORREXION 5 IN 1 DAILY MOISTURIZER SUNSCREEN BROAD SPECTRUM SPF30- avobenzone, homosalate, octisalate, octocrylene lotion
-
Contains inactivated NDC Code(s)
NDC Code(s): 73496-002-01 - Packager: ROC Skincare
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 4, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
PRINCIPAL DISPLAY PANEL
PACKAGE LABEL PRINCIPAL DISPLAY PANEL
OTC - PURPOSE
Active ingredientsPurposeAvobenzone (2%)SunscreenHomosalate (4%)SunscreenOctisalate (4%)SunscreenOctocrylene (2%)Sunscreen

OTC - KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
USES
Helps prevent sunburnif used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
WARNINGS
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
DIRECTIONS
For sunscreen use:apply generously and evenly15 minutes before sun exposurereapply at least every 2 hoursuse a water resistant sunscreen if swimming or sweatingSun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:limit time in the sun, especially from 10 a.m. – 2 p.m.wear long-sleeved shirts, pants, hats, and sunglasseschildren under 6 months of age: Ask a doctor
INACTIVE INGREDIENTS Water, Propylene Glycol, Glycerin, Isononyl Isononanoate, Dimethicone, Steareth-2, Ascorbyl Glucoside, Ethylhexyl Palmitate, Styrene/Acrylates Copolymer, Butyrospermum Parkii (Shea) Butter, Methyl Methacrylate Crosspolymer, Hexylresorcinol, Steareth-21, Behenyl Alcohol, Dimethicone Crosspolymer, Phenoxyethanol, Ammonium Acryloyldimethyltaurate/VP Copolymer, Sodium Hydroxide, Caprylyl Glycol, Sclerotium Gum, Fragrance, Mica, Titanium Dioxide, Methylparaben, Disodium EDTA, Ethylparaben, Propylparaben
-
INGREDIENTS AND APPEARANCE
ROC MULTI CORREXION 5 IN 1 DAILY MOISTURIZER SUNSCREEN BROAD SPECTRUM SPF30
avobenzone, homosalate, octisalate, octocrylene lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73496-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 20 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 40 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 40 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERIN (UNII: PDC6A3C0OX) ISONONYL ISONONANOATE (UNII: S4V5BS6GCX) DIMETHICONE (UNII: 92RU3N3Y1O) STEARETH-2 (UNII: V56DFE46J5) ASCORBYL GLUCOSIDE (UNII: 2V52R0NHXW) ETHYLHEXYL PALMITATE (UNII: 2865993309) STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) SHEA BUTTER (UNII: K49155WL9Y) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) HEXYLRESORCINOL (UNII: R9QTB5E82N) STEARETH-21 (UNII: 53J3F32P58) DOCOSANOL (UNII: 9G1OE216XY) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) PHENOXYETHANOL (UNII: HIE492ZZ3T) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) SODIUM HYDRIDE (UNII: 23J3BHR95O) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BETASIZOFIRAN (UNII: 2X51AD1X3T) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) METHYLPARABEN (UNII: A2I8C7HI9T) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73496-002-01 1 in 1 CARTON 03/01/2013 1 50 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 03/01/2013 Labeler - ROC Skincare (117157981) Registrant - ROC Skincare (117157981) Establishment Name Address ID/FEI Business Operations Janssen Cilag 265148168 manufacture(73496-002)