Label: AMOUR CBD- pain relief cream cream
- NDC Code(s): 72897-001-01
- Packager: SVD Premium Products
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 17, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- PURPOSE
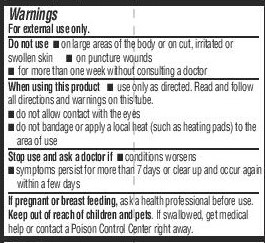
- WARNINGS
- INDICATIONS & USAGE
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
AMOUR CBD
pain relief cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72897-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 mg in 100 g Inactive Ingredients Ingredient Name Strength DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) ALLANTOIN (UNII: 344S277G0Z) AVOCADO OIL (UNII: 6VNO72PFC1) CARBOMER INTERPOLYMER TYPE A (55000 CPS) (UNII: 59TL3WG5CO) ALOE VERA LEAF (UNII: ZY81Z83H0X) CETYL ALCOHOL (UNII: 936JST6JCN) POLYSORBATE 80 (UNII: 6OZP39ZG8H) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) PERILLA FRUTESCENS SEED OIL (UNII: 322MS57V7Z) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) EVENING PRIMROSE OIL (UNII: 3Q9L08K71N) WATER (UNII: 059QF0KO0R) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CANNABIDIOL (UNII: 19GBJ60SN5) MINERAL OIL (UNII: T5L8T28FGP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72897-001-01 44 g in 1 TUBE; Type 0: Not a Combination Product 10/25/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 10/25/2019 Labeler - SVD Premium Products (075268139)