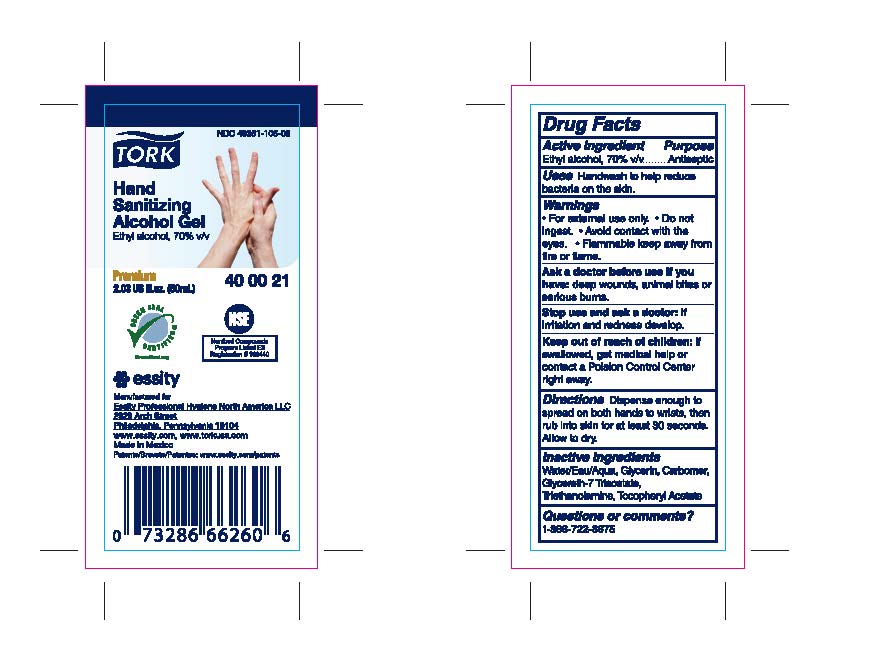
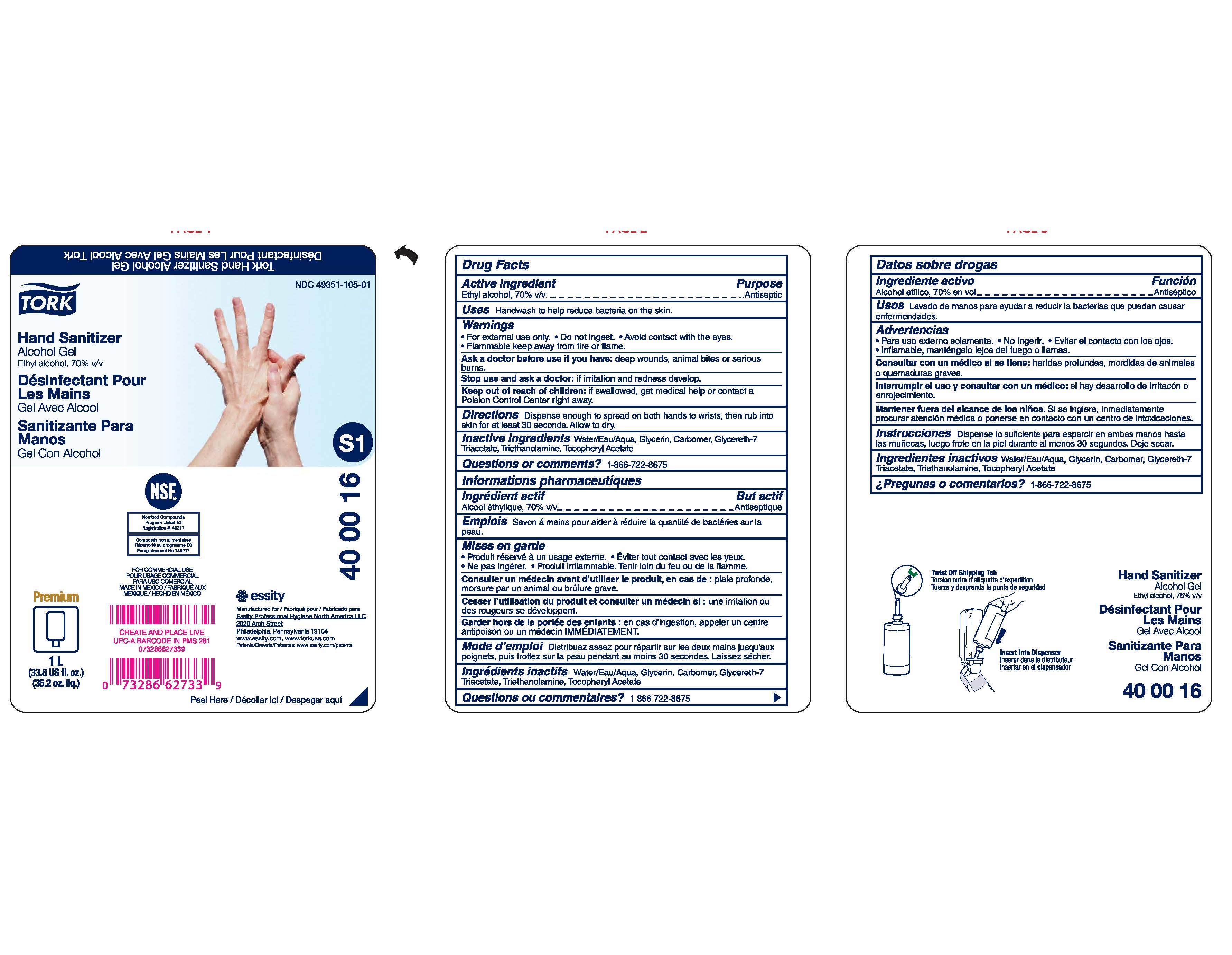
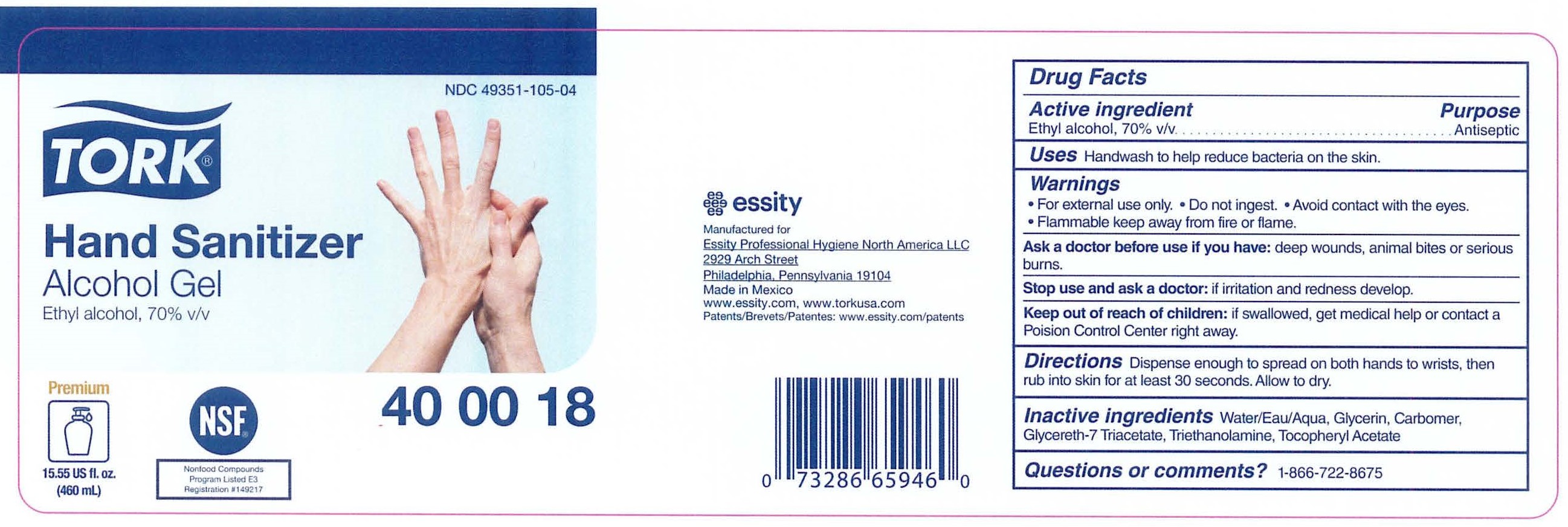
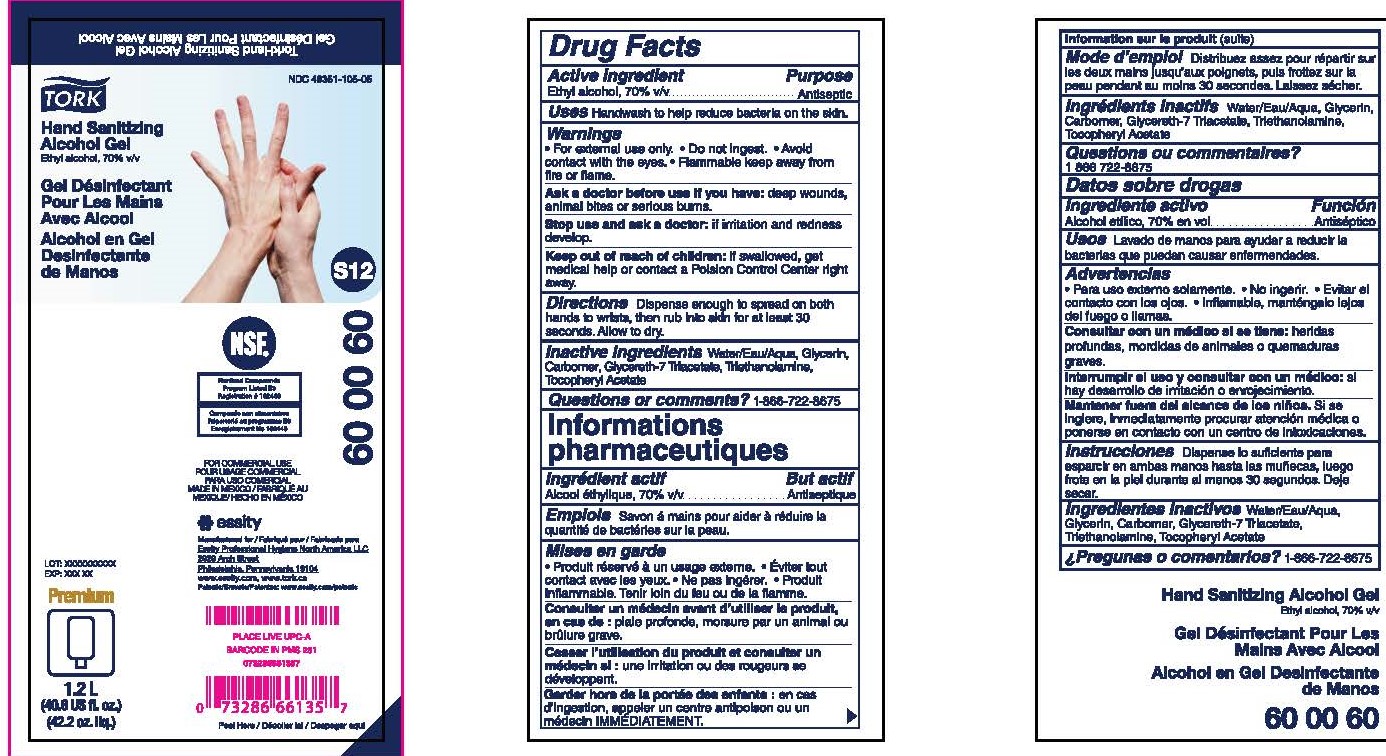
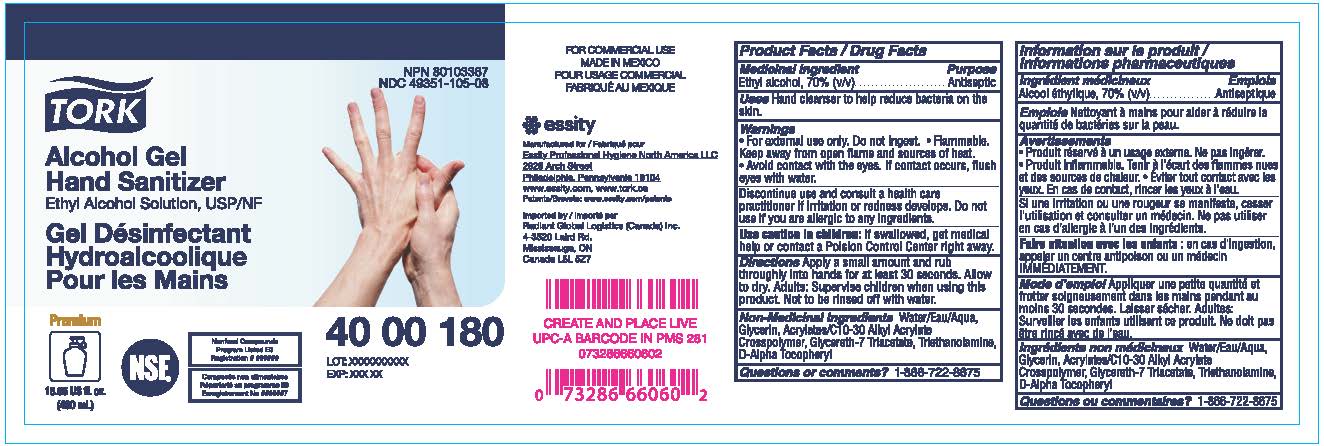
Label: TORK HAND SANITIZER ALCOHOL GEL- ethyl alcohol gel
-
NDC Code(s):
49351-105-01,
49351-105-02,
49351-105-03,
49351-105-04, view more49351-105-05, 49351-105-07, 49351-105-08, 49351-105-09
- Packager: Essity Professional Hygiene North America LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive ingredients
- Questions or Comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TORK HAND SANITIZER ALCOHOL GEL
ethyl alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49351-105 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength GLYCERETH-7 TRIACETATE (UNII: S9W6Z48HUP) WATER (UNII: 059QF0KO0R) TROLAMINE (UNII: 9O3K93S3TK) GLYCERIN (UNII: PDC6A3C0OX) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49351-105-01 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/23/2019 2 NDC:49351-105-02 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/23/2019 3 NDC:49351-105-03 24 in 1 BOX 10/23/2019 12/14/2022 3 240 mL in 1 BOTTLE; Type 0: Not a Combination Product 4 NDC:49351-105-04 12 in 1 BOX 10/23/2019 10/29/2024 4 460 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 5 NDC:49351-105-05 4 in 1 BOX 10/23/2019 07/31/2023 5 1200 mL in 1 BOTTLE; Type 0: Not a Combination Product 6 NDC:49351-105-07 240 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/13/2021 7 NDC:49351-105-08 460 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product 01/13/2021 8 NDC:49351-105-09 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 10/23/2019 03/26/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 10/23/2019 Labeler - Essity Professional Hygiene North America LLC (005694349) Registrant - Cyan Labs S.A. de C.V. (812754130) Establishment Name Address ID/FEI Business Operations Cyan Labs S.A. de C.V. 812754130 manufacture(49351-105) , label(49351-105) , pack(49351-105) , analysis(49351-105)