Label: BESREMI- ropeginterferon alfa-2b injection
- NDC Code(s): 73536-500-01
- Packager: PharmaEssentia USA
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Biologic Licensing Application
Drug Label Information
Updated December 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use BESREMi safely and effectively. See full prescribing information for BESREMi. BESREMI (ropeginterferon alfa-2b-njft) injection, for subcutaneous use Initial U.S. Approval: 2021
WARNING: RISK OF SERIOUS DISORDERS
See full prescribing information for complete boxed warning.
Risk of Serious Disorders: Interferon alfa products may cause or aggravate fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Monitor closely and withdraw therapy with persistently severe or worsening signs or symptoms of the above disorders.
INDICATIONS AND USAGE
BESREMi is an interferon alfa-2b indicated for the treatment of adults with polycythemia vera ( 1)
DOSAGE AND ADMINISTRATION
- Recommended starting dose: 100 mcg by subcutaneous injection every 2 weeks (50 mcg if receiving hydroxyurea).
- Increase the dose by 50 mcg every 2 weeks (up to a maximum of 500 mcg) until hematological parameters are stabilized ( 2.1)
- Interrupt or discontinue dosing if certain adverse reactions occur ( 2.3, 5)
DOSAGE FORMS AND STRENGTHS
- Injection: 500 mcg/mL solution in a single-dose prefilled syringe ( 3)
CONTRAINDICATIONS
- Existence of, or history of severe psychiatric disorders, particularly severe depression, suicidal ideation or suicide attempt ( 4)
- Hypersensitivity to interferon or to any component of BESREMi ( 4)
- Hepatic impairment (Child-Pugh B or C) ( 4)
- History or presence of active serious or untreated autoimmune disease ( 4)
- Immunosuppressed transplant recipients ( 4)
WARNINGS AND PRECAUTIONS
Patients exhibiting the following events should be closely monitored and may require dose reduction or discontinuation of therapy:
- Depression and Suicide: Monitor closely for symptoms and need for treatment. ( 5.1)
- Endocrine Toxicity: Discontinue if endocrine disorders occur that cannot be medically managed. ( 5.2)
- Cardiovascular Toxicity: Avoid use in patients with severe, acute or unstable cardiovascular disease. Monitor patients with history of cardiovascular disorders more frequently. ( 5.3)
- Decreased Peripheral Blood Counts: Perform blood counts at baseline, every 2 weeks during titration, and at least every 3-6 months during maintenance treatment. ( 5.4)
- Hypersensitivity Reactions: Stop treatment and immediately manage reaction. ( 5.5)
- Pancreatitis: Consider discontinuation if confirmed pancreatitis ( 5.6)
- Colitis: Discontinue if signs or symptoms of colitis ( 5.7)
- Pulmonary Toxicity: Discontinue if pulmonary infiltrates or pulmonary function impairment ( 5.8)
- Ophthalmologic Toxicity: Advise patients to have eye examinations before and during treatment. Evaluate eye symptoms promptly and discontinue if new or worsening eye disorders. ( 5.9)
- Hyperlipidemia: Monitor serum triglycerides before BESREMi treatment and intermittently during therapy and manage when elevated. ( 5.10)
- Hepatotoxicity: Monitor liver enzymes and hepatic function at baseline and during treatment. Reduce dose or discontinue depending on severity. ( 5.11)
- Renal Toxicity: Monitor serum creatinine at baseline and during therapy. Discontinue if severe renal impairment develops. ( 5.12)
- Dental and Periodontal Toxicity: Advise patients on good oral hygiene and to have regular dental examinations. ( 5.13)
- Dermatologic Toxicity: Consider discontinuing if clinically significant dermatologic toxicity. ( 5.14)
- Driving and Operating Machinery: Advise patients to avoid driving or using machinery if they experience dizziness, somnolence, or hallucination. ( 5.15)
ADVERSE REACTIONS
The most common adverse reactions reported in > 40% of patients were influenza-like illness, arthralgia, fatigue, pruritus, nasopharyngitis, and musculoskeletal pain ( 6).
To report SUSPECTED ADVERSE REACTIONS, contact PharmaEssentia at 1-800-999-2449 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Monitor patients taking CYP450 substrates with a narrow therapeutic index for adverse reactions to inform the need for dose adjustment of the concomitant drug ( 7.1)
- Avoid use with myelosuppressive agents and monitor patients receiving the combination for effects of excessive myelosuppression ( 7.2)
- Avoid use with narcotics, hypnotics or sedatives. Monitor patients receiving the combination for excessive central nervous system toxicity ( 7.3)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. ( 8.1, 8.3)
- Lactation: Advise women not to breastfeed during treatment and for 8 weeks after the final dose. ( 8.2)
- Avoid use in patients with eGFR <30 mL/min. ( 8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2021
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
These highlights do not include all the information needed to use BESREMi safely and effectively. See full prescribing information for BESREMi. BESREMi (ropeginterferon alfa-2b-njft) injection, for subcutaneous use Initial U.S. Approval: 2021
WARNING: RISK OF SERIOUS DISORDERS1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Pre-Treatment Testing
2.2 Recommended Dosage
2.3 Dose Modifications
2.4 Preparation and Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Depression and Suicide
5.2 Endocrine Toxicity
5.3 Cardiovascular Toxicity
5.4 Decreased Peripheral Blood Counts
5.5 Hypersensitivity Reactions
5.6 Pancreatitis
5.7 Colitis
5.8 Pulmonary Toxicity
5.9 Ophthalmologic Toxicity
5.10 Hyperlipidemia
5.11 Hepatotoxicity
5.12 Renal Toxicity
5.13 Dental and Periodontal Toxicity
5.14 Dermatologic Toxicity
5.15 Driving and Operating Machinery
5.16 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Immunogenicity
7 DRUG INTERACTIONS
7.1 Drugs Metabolized by Cytochrome P450
7.2 Myelosuppressive Agents
7.3 Narcotics, Hypnotics or Sedatives
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
These highlights do not include all the information needed to use BESREMi safely and effectively. See full prescribing information for BESREMi. BESREMi (ropeginterferon alfa-2b-njft) injection, for subcutaneous use Initial U.S. Approval: 2021
WARNING: RISK OF SERIOUS DISORDERSRisk of Serious Disorders: Interferon alfa products may cause or aggravate fatal or life-threatening neuropsychiatric, autoimmune, ischemic, and infectious disorders. Patients should be monitored closely with periodic clinical and laboratory evaluations. Therapy should be withdrawn in patients with persistently severe or worsening signs or symptoms of these conditions. In many, but not all cases, these disorders resolve after stopping therapy [ see Warnings and Precautions (5.1, 5,2, 5.3, 5.4) and Adverse Reactions (6.1)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Pre-Treatment Testing
Pregnancy testing is recommended prior to BESREMi treatment in females of reproductive potential [see Use in Specific Populations (8.3)] .
2.2 Recommended Dosage
Patients Not Already on Hydroxyurea:
- The recommended BESREMi starting dosage for patients not on hydroxyurea is 100 mcg by subcutaneous injection every two weeks.
- Increase the dose by 50 mcg every two weeks (up to a maximum of 500 mcg), until the hematological parameters are stabilized (hematocrit less than 45%, platelets less than 400 × 10 9/L, and leukocytes less than 10 × 10 9/L).
Patients Transitioning from Hydroxyurea:
- When transitioning to BESREMi from hydroxyurea, start BESREMi at 50 mcg by subcutaneous injection every two weeks in combination with hydroxyurea.
- Gradually taper off the hydroxyurea by reducing the total biweekly dose by 20-40% every two weeks during Weeks 3-12.
- Increase the dose of BESREMi by 50 mcg every two weeks (up to a maximum of 500 mcg), until the hematological parameters are stabilized (hematocrit less than 45%, platelets less than 400 × 10 9/L, and leukocytes less than 10 × 10 9/L).
- Discontinue hydroxyurea by Week 13.
Maintain the two week dosing interval of BESREMi at which hematological stability is achieved for at least 1 year. After achievement of hematological stability for at least 1 year on a stable dose of BESREMi, the dosing interval may be expanded to every 4 weeks.
Monitor patients closely especially during the titration phase. Perform complete blood counts (CBC) regularly, every 2 weeks during the titration phase and every 3-6 months during the maintenance phase (after the patient's optimal dose is established). Monitor CBC more frequently if clinically indicated. Phlebotomy as rescue treatment to normalize blood hyperviscosity may be necessary during the titration phase [see Clinical Pharmacology (12.2)] .
2.3 Dose Modifications
Monitor CBC every 2 weeks during the titration phase and dose modification phase. Phlebotomy as rescue treatment to normalize blood hyperviscosity may be necessary [see Clinical Pharmacology (12.2)] .
If dose interruption occurs, resume dosing at previously attained levels. If drug-related toxicities arise, reduce the dose to the next lower level or interrupt in accordance with the table below (Table 1). If there is insufficient efficacy at the decreased dose following dose modification, a dose increase attempt to the next higher dose level should be considered after recovery to grade 1 toxicity.
Table 1 Dose Modifications for BESREMi Adverse Reactions Adverse Reaction * Severity Dosage Modification - *
- National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE), version 3.0
Liver enzyme elevation with concomitant bilirubin elevation, or other evidence of hepatic decompensation Any increase above baseline Interrupt treatment until recovery, restart at dose 50 mcg lower than the interrupted dose. If the interrupted dose is 50 mcg, refrain from treatment until recovery. Consider permanent discontinuation if toxicity persists after four dose-modifications. Liver enzyme elevation >5 × the upper limit of normal (ULN) but ≤20 × ULN Decrease dose by 50 mcg; if toxicity does not improve, continue decreasing at biweekly intervals until alanine aminotransferase (ALT) and aspartate aminotransferase (AST) recover < 3 × ULN if baseline was normal; 3 × baseline if baseline was abnormal, and gamma-glutamyltransferase (GGT) recovers to < 2.5 × ULN if baseline was normal; 2.5 × baseline if baseline was abnormal. If the interrupted dose is 50 mcg, refrain from treatment until recovery. >20 × ULN Interrupt treatment until ALT and AST recover to < 3 × ULN if baseline was normal; 1.5 × baseline if baseline was abnormal, and gamma-glutamyltransferase (GGT) recovers to < 2.5 × ULN if baseline was normal; 2 × baseline if baseline was abnormal. Consider permanent discontinuation if toxicity persists after four dose-modifications. Cytopenia Anemia: Hemoglobin (Hgb) < 8 g/dL Decrease dose by 50 mcg; if toxicity does not improve, continue decreasing at biweekly intervals until recovery of Hgb >10.0 g/dL, platelets >75,000/mm 3, and WBC >3,000/mm 3 Thrombocytopenia: platelet count < 50,000/mm 3 but ≥25,000/mm 3 Leukopenia: white blood cell count (WBC) <2000/mm 3 but ≥1,000/mm 3 If the interrupted dose is 50 mcg, refrain from treatment until recovery. Anemia: Hemoglobin levels are life threatening, or urgent intervention needed Interrupt treatment until recovery of Hgb >10.0 g/dL, platelets >75,000/mm 3, and WBC >3,000/mm 3. Thrombocytopenia: platelet count <25,000/mm 3 Consider permanent discontinuation if toxicity persists after four dose-modifications. Leukopenia: WBC <1000/mm 3 Depression Mild, without suicidal ideation Consider psychiatric consultation if persistent (>8 weeks). Moderate, without suicidal ideation Consider dose reduction and psychiatric consultation. Severe, or any severity with suicidal ideation Discontinue therapy, recommend psychiatric consultation. 2.4 Preparation and Administration
Read the INSTRUCTIONS FOR USE before administering the single-dose BESREMi prefilled syringe. BESREMi is for subcutaneous injection only and may be administered by either a healthcare professional, a patient or a caregiver. Before a decision is made to allow BESREMi to be administered by a patient or caregiver, ensure that the patient is an appropriate candidate for self-administration or administration by a caregiver. Proper training on storage, preparation and administration technique should be provided. If a patient or caregiver is not an appropriate candidate for any reason, then BESREMi should be administered by a healthcare professional.
Before each injection, remove the carton that contains the BESREMi prefilled syringe from the refrigerator. Keep the prefilled syringe in the carton and lay it flat on a clean work surface for 15-30 minutes to allow the prefilled syringe to reach room temperature [59 ˚F to 77 ˚F (15 ˚C to 25 ˚C)].
Before injection, visually inspect BESREMi in the prefilled syringe for particulate matter and discoloration before administration (do not use if the solution in the syringe is cloudy, discolored, contains particulate matter or if the syringe shows any sign of damage).
Syringe Preparation
- Remove the prefilled syringe cap by unscrewing it counterclockwise.
- Attach the covered needle to the prefilled syringe by firmly pushing it onto the collar of the syringe and then screwing (turn clockwise) it on until it feels securely attached.
- Choose one of the following injection sites: Lower stomach (abdomen) area, at least 2 inches away from the belly button, or top of thighs. Rotate (change) the injection site for each injection. Do not inject into skin that is irritated, red, bruised, infected, or scarred; clean the chosen injection site with an alcohol swab and let air dry.
- Uncap needle and move air bubbles to top. Pull the pink needle shield back and hold the syringe from the syringe body. Remove the clear needle cap by pulling it straight off. Throw away the needle cap into the trash. Hold the prefilled syringe with the needle pointing up. Tap on the body of the prefilled syringe to move any air bubbles to the top.
Set Injection Dose
- Depending on the prescribed dose, the amount of dose in the syringe may need to be adjusted by discarding some of the medication.
- Hold the prefilled syringe at eye level with the needle pointing straight up over a paper towel, sink, or trash can. Check that you can see the dose lines and number markings on the prefilled syringe.
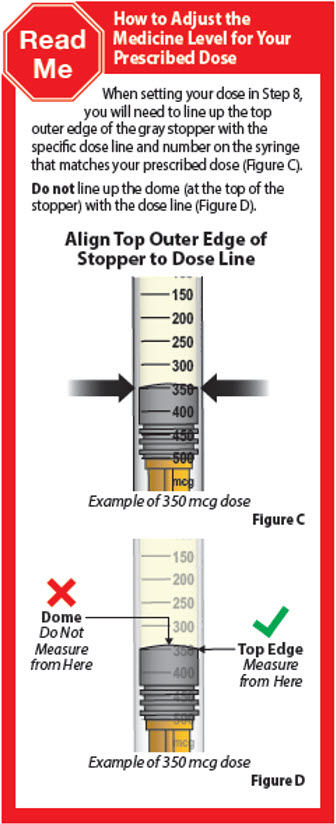
- Pinch the end of the plunger and slowly push up to remove liquid medicine until the top edge of the gray stopper lines up with the marking for the prescribed dose.
Inject BESREMi
- Pinch the chosen injection site. While pinching the skin, insert needle at a 45- to 90-degree angle into the pinched skin, then release the pinched skin.
- Inject BESREMi by slowly pressing on the plunger all the way until it stops. After all the liquid medicine is injected, remove the needle from the skin.
Dispose of Used Syringe
- Carefully push the pink needle shield over the needle until it snaps into place and covers the needle. Do not recap the needle using the needle cap; only use the pink needle shield to cover the needle.
- Throw away the used prefilled syringe with the needle still attached, into an FDA-cleared sharps disposal container.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
BESREMi is contraindicated in patients with:
- Existence of, or history of severe psychiatric disorders, particularly severe depression, suicidal ideation, or suicide attempt
- Hypersensitivity to interferons including interferon alfa-2b or any of the inactive ingredients of BESREMi
- Moderate (Child-Pugh B) or severe (Child-Pugh C) hepatic impairment
- History or presence of active serious or untreated autoimmune disease
- Immunosuppressed transplant recipients
-
5 WARNINGS AND PRECAUTIONS
5.1 Depression and Suicide
Life-threatening or fatal neuropsychiatric reactions have occurred in patients receiving interferon alfa products, including BESREMi. These reactions may occur in patients with and without previous psychiatric illness. Serious neuropsychiatric reactions have been observed in 3% of patients treated with BESREMi during the clinical development program. Among the 178 patients in the clinical development program of BESREMi, 17 cases of depression, depressive symptoms, depressed mood, and listlessness occurred. Of these seventeen cases, 3.4% of the patients recovered with temporary drug interruption and 2.8% stopped BESREMi treatment.
Other central nervous system effects, including suicidal ideation, attempted suicide, aggression, bipolar disorder, mania and confusion have been observed with other interferon alfa products. BESREMi is contraindicated in patients with a history of severe psychiatric disorders, particularly severe depression, suicidal ideation, or suicide attempt [see Contraindications (4)] .
Closely monitor patients for any symptoms of psychiatric disorders and consider psychiatric consultation and treatment if such symptoms emerge. If psychiatric symptoms worsen, it is recommended to discontinue BESREMi therapy.
5.2 Endocrine Toxicity
Endocrine toxicity has occurred in patients receiving interferon alfa products, including BESREMi. These toxicities may include worsening hypothyroidism and hyperthyroidism. Autoimmune thyroiditis and hyperglycemia, including new onset type 1 diabetes, have been reported in patients receiving interferon alfa-2b products. Eight cases of hyperthyroidism (4.5%), seven cases of hypothyroidism (3.9%) and five cases (2.8%) of autoimmune thyroiditis/thyroiditis occurred in the development program of BESREMi.
Do not use BESREMi in patients with active serious or untreated endocrine disorders associated with autoimmune disease [Contraindications (4)]. Evaluate thyroid function in patients who develop symptoms suggestive of thyroid disease during BESREMi therapy. Discontinue BESREMi in patients who develop endocrine disorders that cannot be adequately managed during treatment with BESREMi.
5.3 Cardiovascular Toxicity
Cardiovascular toxicity has occurred in patients receiving interferon alfa products, including BESREMi. Toxicities may include cardiomyopathy, myocardial infarction, atrial fibrillation and coronary artery ischemia [see Adverse Reactions (6.1)] . Patients with a history of cardiovascular disorders should be closely monitored for cardiovascular toxicity during BESREMi therapy. Avoid use of BESREMi in patients with severe or unstable cardiovascular disease, (e.g., uncontrolled hypertension, congestive heart failure (≥ NYHA class 2), serious cardiac arrhythmia, significant coronary artery stenosis, unstable angina) or recent stroke or myocardial infarction.
5.4 Decreased Peripheral Blood Counts
Decreased peripheral blood counts have occurred in patients receiving interferon alfa products, including BESREMi. These toxicities may include thrombocytopenia (increasing the risk of bleeding), anemia, and leukopenia (increasing the risk of infection). Thrombocytopenia of grade 3 (platelet counts <50,000 – 25,000/mm 3) or greater occurred in 2% of BESREMi-treated patients. Anemia of grade 3 (Hgb < 8 g/dL) or greater occurred in 1% of BESREMi-treated patients. Leukopenia of grade 3 (WBC counts <2,000 – 1,000/mm 3) or greater occurred in 2% of BESREMi-treated patients. Infection occurred in 48% of BESREMi treated patients, while serious infections occurred in 8% of BESREMi treated patients. Monitor complete blood counts at baseline, during titration and every 3-6 months during the maintenance phase. Monitor patients for signs and symptoms of infection or bleeding.
5.5 Hypersensitivity Reactions
Hypersensitivity reactions have occurred in patients receiving interferon alfa products, including BESREMi. BESREMi is contraindicated in patients with hypersensitivity reactions to interferon products or any of the inactive ingredients in BESREMi [see Contraindications (4)] . Toxicities may include serious, acute hypersensitivity reactions (e.g., urticaria, angioedema, bronchoconstriction, anaphylaxis). If such reactions occur, discontinue BESREMi and institute appropriate medical therapy immediately. Transient rashes may not necessitate interruption of treatment.
5.6 Pancreatitis
Pancreatitis has occurred in patients receiving interferon alfa products, including BESREMi. Pancreatitis was reported in 2.2% of patients receiving BESREMi. Symptoms may include nausea, vomiting, upper abdominal pain, bloating, and fever. Patients may experience elevated lipase, amylase, white blood cell count, or altered renal/hepatic function. Interrupt BESREMi treatment in patients with possible pancreatitis and evaluate promptly. Consider discontinuation of BESREMi in patients with confirmed pancreatitis.
5.7 Colitis
Fatal and serious ulcerative or hemorrhagic/ischemic colitis have occurred in patients receiving interferon alfa products, some cases occurring as early as 12 weeks after start of treatment. Symptoms may include abdominal pain, bloody diarrhea, and fever. Discontinue BESREMi in patients who develop these signs or symptoms. Colitis may resolve within 1 to 3 weeks of stopping treatment.
5.8 Pulmonary Toxicity
Pulmonary toxicity has occurred in patients receiving interferon alfa products, including BESREMi. Pulmonary toxicity may manifest as dyspnea, pulmonary infiltrates, pneumonia, bronchiolitis obliterans, interstitial pneumonitis, pulmonary hypertension, and sarcoidosis. Some events have resulted in respiratory failure or death. Discontinue BESREMi in patients who develop pulmonary infiltrates or pulmonary function impairment.
5.9 Ophthalmologic Toxicity
Ophthalmologic toxicity has occurred in patients receiving interferon alfa products, including BESREMi. These toxicities may include severe eye disorders such as retinopathy, retinal hemorrhage, retinal exudates, retinal detachment and retinal artery or vein occlusion which may result in blindness. During BESREMi therapy, 23% of patients were identified with an eye disorder. Eyes disorders ≥5% included cataract (6%) and dry eye (5%). Advise patients to have eye examinations before and during BESREMi therapy, specifically in those patients with a retinopathy-associated disease such as diabetes mellitus or hypertension. Evaluate eye symptoms promptly. Discontinue BESREMi in patients who develop new or worsening eye disorders.
5.10 Hyperlipidemia
Hyperlipidemia has occurred in patients treated with interferon alfa products, including BESREMi. Hyperlipidemia, hypertriglyceridemia, or dyslipidemia occurred in 3% of patients receiving BESREMi. Elevated triglycerides may result in pancreatitis [see Warnings and Precautions (5.6)] . Monitor serum triglycerides before BESREMi treatment and intermittently during therapy and manage when elevated. Consider discontinuation of BESREMi in patients with persistently, markedly elevated triglycerides.
5.11 Hepatotoxicity
Hepatotoxicity has occurred in patients receiving interferon alfa products, including BESREMi. These toxicities may include increases in serum ALT, AST, GGT and bilirubin. BESREMi is contraindicated in patients with moderate (Child-Pugh B) or severe (Child-Pugh C) hepatic impairment [see Contraindications (4)] .
Increases in serum ALT ≥3 times the upper limit of normal (ULN), AST ≥3 times the ULN, GGT ≥3 times the ULN, and bilirubin >2 times the ULN have been observed in patients treated with BESREMi.
In the clinical development program of BESREMi, 36 patients (20%) experienced liver enzyme elevations, 33 of whom had elevations of 1.25-5× ULN. Patients were able to resume BESREMi upon resolution of liver enzyme elevations. Liver enzyme elevations have also been reported in patients after long-term BESREMi therapy.
Monitor liver enzymes and hepatic function at baseline and during BESREMi treatment. Reduce BESREMi dosage by 50 mcg for increased AST/ALT/GGT then monitor AST/ALT/GGT weekly until the values return to baseline or grade 1 (ALT and AST < 3 × ULN if baseline was normal; 1.5 - 3 × baseline if baseline was abnormal, and GGT < 2.5 × ULN if baseline was normal; 2 - 2.5 × baseline if baseline was abnormal) [see Dosage and Administration (2.3)]. If toxicity does not improve, continue decreasing the BESREMi dose at biweekly intervals until recovery to grade 1. Hold if AST/ALT/GGT > 20 × ULN and consider permanent discontinuation if increased liver enzyme levels persist after four dose-reductions. Discontinue BESREMi in patients who develop evidence of hepatic decompensation (characterized by jaundice, ascites, hepatic encephalopathy, hepatorenal syndrome or variceal hemorrhage) during treatment [see Use in Specific Populations (8.7)] .
5.12 Renal Toxicity
Renal toxicity has occurred in patients receiving interferon alfa products, including BESREMi. During BESREMi therapy, <1% of patients were reported to develop renal impairment and <1% of patients were reported to have toxic nephropathy. Monitor serum creatinine at baseline and during therapy. Avoid use of BESREMi in patients with eGFR <30 mL/min. Discontinue BESREMi if severe renal impairment develops during treatment [see Use in Specific Populations (8.6)] .
5.13 Dental and Periodontal Toxicity
Dental and periodontal toxicities may occur in patients receiving interferon alfa products, including BESREMi. These toxicities may include dental and periodontal disorders, which may lead to loss of teeth. In addition, dry mouth could have a damaging effect on teeth and oral mucous membranes during long-term treatment with BESREMi. Patients should have good oral hygiene and regular dental examinations.
5.14 Dermatologic Toxicity
Dermatologic toxicity has occurred in patients receiving interferon alfa products, including BESREMi. These toxicities have included skin rash, pruritus, alopecia, erythema, psoriasis, xeroderma, dermatitis acneiform, hyperkeratosis, and hyperhidrosis. Consider discontinuation of BESREMi if clinically significant dermatologic toxicity occurs.
5.15 Driving and Operating Machinery
BESREMi may impact the ability to drive and use machinery. Patients should not drive or use heavy machinery until they know how BESREMi affects their abilities. Patients who experience dizziness, somnolence or hallucination during BESREMi therapy should avoid driving or using machinery.
5.16 Embryo-Fetal Toxicity
Based on the mechanism of action, BESREMi can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1) and Use in Specific Populations (8.1)] . Pregnancy testing is recommended in females of reproductive potential prior to treatment with BESREMi. Advise females of reproductive potential to use an effective method of contraception during treatment with BESREMi and for at least 8 weeks after the final dose [see Dosage and Administration (2.1) and Use in Specific Populations (8.1, 8.3)] .
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
The following clinically significant adverse reactions are described elsewhere in the labeling.
- Depression and Suicide [see Warnings and Precautions (5.1)]
- Endocrine Toxicity [see Warnings and Precautions (5.2)]
- Cardiovascular Toxicity [see Warnings and Precautions (5.3)]
- Decreased Peripheral Blood Counts [see Warnings and Precautions (5.4)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.5)]
- Pancreatitis [see Warnings and Precautions (5.6)]
- Colitis [see Warnings and Precautions (5.7)]
- Pulmonary Toxicity [see Warnings and Precautions (5.8)]
- Ophthalmologic Toxicity [see Warnings and Precautions (5.9)]
- Hyperlipidemia [see Warnings and Precautions (5.10)]
- Hepatotoxicity [see Warnings and Precautions (5.11)]
- Renal Toxicity [see Warnings and Precautions (5.12)]
- Dental and Periodontal Toxicity [see Warnings and Precautions (5.13)]
- Dermatologic Toxicity [see Warnings and Precautions (5.14)]
- Driving and Operating Machinery [see Warnings and Precautions (5.15)]
- Embryo-Fetal Toxicity [see Warnings and Precautions (5.16)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The pooled safety population described in the Warnings and Precautions section reflects exposure to BESREMi as monotherapy for the treatment of polycythemia vera dosed every two to four weeks in 178 patients in two open-label trials [ PEGINVERA, PROUD/CONTINUATION PV]. The mean age at baseline was 58.6 years (range 30-85 years), 88 (49.4%) women, 90 (50.6%) men, 177 (99%) Caucasian and 1 (1%) Asian. Among 178 patients who received BESREMi, 80% were exposed for 12 months or longer. The mean dose of BESREMi was 334 mcg SD ± 121 during the treatment period. In this pooled safety population, the most common adverse reactions greater than 10%, were liver enzyme elevations (20%), leukopenia (20%), thrombocytopenia (19%), arthralgia (13%), fatigue (12%), myalgia (11%), and influenza-like illness (11%).
The safety findings described below reflect exposure to BESREMi as monotherapy for the treatment of polycythemia vera in 51 patients in the PEGINVERA study [see Clinical Studies (14)] . Among the 51 patients receiving BESREMi, 71% were exposed for 12 months or longer, 63% were exposed for three years or longer, and 53% were exposed for greater than five years.
Serious adverse reactions were reported in 16% of patients in the PEGINVERA study. The most common serious adverse reactions observed during the study (≥ 4%) included urinary tract infection (8%), transient ischemic attack (6%) and depression (4%).
Adverse reactions requiring permanent discontinuation in >2% of patients who received BESREMi included depression (8%), arthralgia (4%), fatigue (4%), and general physical health deterioration (4%) In the PEGINVERA study, patients were not pre-screened for depression or anxiety disorders.
The most common adverse reactions reported in ≥10% of patients in the PEGINVERA study are listed in Table 2.
Table 2 Adverse Reactions in > 10% of Subjects with Polycythemia Vera in the PEGINVERA Study Over 7.5 Years. Adverse Reactions * BESREMi
N=51
%Grouped Term Definitions - *
- Adverse Reactions defined as all treatment emergent adverse events
- †
- Includes pyrexia, chills, and influenza-like illness.
- ‡
- Includes asthenia, malaise, and fatigue.
- §
- Includes pharyngitis and nasopharyngitis.
- ¶
- Includes musculoskeletal pain, back pain, pain in extremity, bone pain, flank pain, and spinal pain.
- #
- Includes headache, migraine, and head pain.
- Þ
- Includes night sweats and hyperhidrosis.
- ß
- Includes upper respiratory tract infection, rhinitis, bronchitis, and respiratory tract infection.
- à
- Includes abdominal pain upper, abdominal pain lower, and abdominal pain.
- è
- Includes insomnia, sleep disorder, and abnormal dreams.
- ð
- Includes peripheral edema and generalized edema.
- ø
- Includes hypertension and hypertensive crisis.
- ý
- Includes rash, maculopapular rash, and pruritic rash.
- £
- Includes transaminase increase, hepatic enzyme increase, GGT increase, AST increase, and ALT increase.
Influenza-like illness † 59 Arthralgia 47 Fatigue ‡ 47 Pruritis 45 Nasopharyngitis § 43 Musculoskeletal pain ¶ 41 Headache # 39 Diarrhea 33 Hyperhidrosis Þ 29 Nausea 28 Upper respiratory tract infection ß 27 Local administration site reactions 26 Dizziness 22 Abdominal pain à 20 Depression 20 Sleep disorder è 20 Leukopenia 18 Decreased appetite 18 Alopecia 16 Edema ð 16 Hypertension ø 16 Muscle spasms 16 Neutropenia 16 Rash ý 16 Transaminase elevations £ 16 Urinary tract infection 16 Thrombocytopenia 12 Vertigo 12 Clinically relevant adverse reactions in < 10% of patients include:
Cardiovascular System: Atrial fibrillation
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other interferon alfa-2b products may be misleading.
The incidence of binding antibodies to ropeginterferon alfa-2b-njft was 1.4% (2/146) and they were observed as early as 8 weeks post-dosing. Among the patients who tested positive for binding antibodies, none developed neutralizing antibodies.
-
7 DRUG INTERACTIONS
7.1 Drugs Metabolized by Cytochrome P450
Certain proinflammatory cytokines, including interferons, can suppress CYP450 enzymes resulting in increased exposures of some CYP substrates [see Clinical Pharmacology (12.3)] . Therefore, patients on BESREMi who are receiving concomitant drugs that are CYP450 substrates with a narrow therapeutic index should be monitored to inform the need for dosage modification for these concomitant drugs.
7.2 Myelosuppressive Agents
Concomitant use of BESREMi and myelosuppressive agents can produce additive myelosuppression. Avoid use and monitor patients receiving the combination for effects of excessive myelosuppression [see Warnings and Precautions (5.4)] .
7.3 Narcotics, Hypnotics or Sedatives
Concomitant use of BESREMi and narcotics, hypnotics or sedatives can produce additive neuropsychiatric side effects. Avoid use and monitor patients receiving the combination for effects of excessive CNS toxicity [see Warnings and Precautions (5.1)] .
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available human data with BESREMi use in pregnant women are insufficient to identify a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal studies assessing reproductive toxicity of BESREMi have not been conducted. Based on mechanism of action and the role of interferon alfa in pregnancy and fetal development, BESREMi may cause fetal harm and should be assumed to have abortifacient potential when administered to a pregnant woman. There are adverse effects on maternal and fetal outcomes associated with polycythemia vera in pregnancy (see Clinical Considerations) . Advise pregnant women of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage is 2-4% and 15-20%, respectively.
8.2 Lactation
There are no data on the presence of BESREMi in human or animal milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in breastfed children from BESREMi, advise women not to breastfeed during treatment and for 8 weeks after the final dose.
8.3 Females and Males of Reproductive Potential
BESREMi may cause embryo-fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)] .
Pregnancy Testing
Pregnancy testing prior to BESREMi treatment is recommended for females of reproductive potential.
Infertility
Females
Based on its mechanism of action, BESREMi can cause disruption of the menstrual cycle [see Clinical Pharmacology (12.1)] . No animal fertility studies have been conducted with BESREMi.
8.5 Geriatric Use
Clinical studies of BESREMi did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other therapy.
8.6 Renal Impairment
No dose adjustment is necessary in patients with estimated glomerular filtration rate (eGFR) ≥30 mL/min [see Clinical Pharmacology (12.3)]. Avoid use of BESREMi in patients with eGFR <30 mL/min [see Warnings and Precautions (5.12)].
8.7 Hepatic Impairment
BESREMi is contraindicated in patients with hepatic impairment (Child-Pugh B or C) [see Contraindications (4)] .
Increased liver enzyme levels have been observed in patients treated with BESREMi. When the increase in liver enzyme levels is progressive and persistent, reduce the dose of BESREMi. If the increase in liver enzymes is progressive and clinically significant despite dose-reduction, or if there is evidence of hepatic impairment (Child-Pugh B or C), discontinue BESREMi [see Dosage and Administration (2.2) and Warnings and Precautions (5.11)] .
- 10 OVERDOSAGE
-
11 DESCRIPTION
Ropeginterferon alfa-2b-njft, an interferon alfa-2b, is an N-terminal monopegylated covalent conjugate of proline interferon alfa-2b, produced in Escherichia coli cells by recombinant DNA technology, with a methoxy polyethylene glycol (mPEG) moiety. Ropeginterferon alfa-2b-njft has an approximate molecular weight of 60 kDa and the approximate molecular weight of the PEG portion of the molecule is 40 kDa.
BESREMi (ropeginterferon alfa-2b-njft) injection is a sterile, preservative-free, clear and colorless to slightly yellowish solution for subcutaneous use supplied in a single dose prefilled syringe.
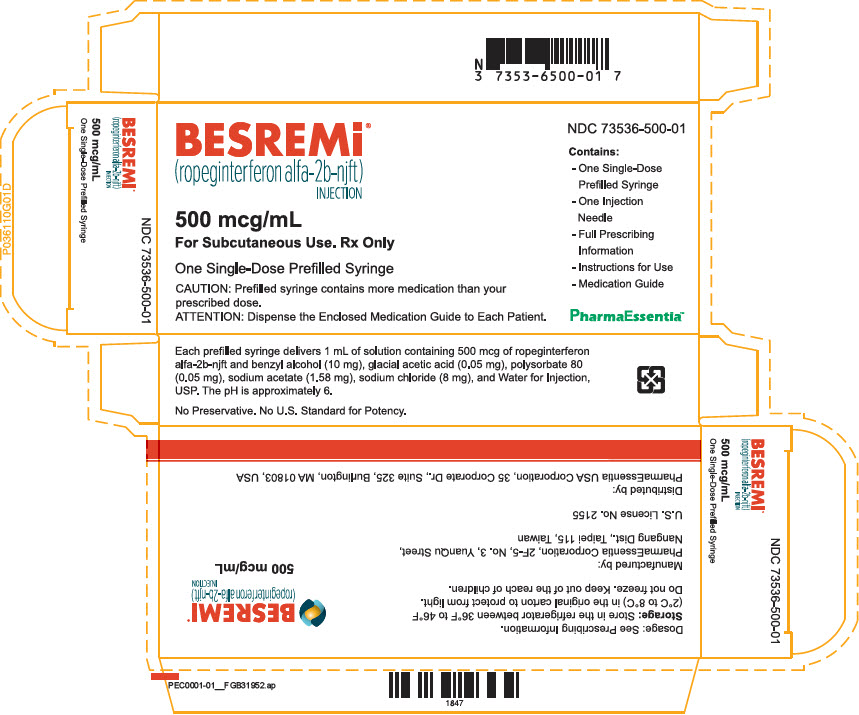
Each prefilled syringe delivers 1 mL of solution containing 500 mcg of ropeginterferon alfa-2b-njft and benzyl alcohol (10 mg), glacial acetic acid (0.05 mg), polysorbate 80 (0.05 mg), sodium acetate (1.58 mg), sodium chloride (8 mg), and Water for Injection, USP. The pH is approximately 6.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Interferon alfa belongs to the class of type I interferons, which exhibit their cellular effects in polycythemia vera in the bone marrow by binding to a transmembrane receptor termed interferon alfa receptor (IFNAR). Binding to IFNAR initiates a downstream signaling cascade through the activation of kinases, in particular Janus kinase 1 (JAK1) and tyrosine kinase 2 (TYK2) and activator of transcription (STAT) proteins. Nuclear translocation of STAT proteins controls distinct gene-expression programs and exhibits various cellular effects. The actions involved in the therapeutic effects of interferon alfa in polycythemia vera are not fully elucidated.
12.2 Pharmacodynamics
The efficacy of ropeginterferon alfa-2b-njft is dependent on the stabilization of hematological parameters (hematocrit <45%, platelets <400 × 10 9/L and leukocytes <10 × 10 9/L). Pharmacokinetic-pharmacodynamic analyses have demonstrated that the reduction in the individual hematological parameters is dependent on ropeginterferon alfa-2b-njft concentrations. Complete hematological response (CHR, defined as a patient achieving hematocrit <45% without phlebotomy [at least 2 months since last phlebotomy], platelets ≤400 × 10 9/L and leukocytes ≤10 × 10 9/L) increased with increasing ropeginterferon alfa-2b-njft concentration over time. Based on the exposure-response (E-R) analyses using data from the PEGINVERA study, the predicted probability of CHR (95% Prediction Intervals) was 22% (11% – 34%) before treatment, 50% (38% – 62%) at week 20 (end of titration), 64% (47% – 78%) at week 52, and 70% (55% – 88%) at week 104. The E-R analyses show that the maximum probability of CHR is reached after 2 years of continuous treatment.
12.3 Pharmacokinetics
In patients with polycythemia vera, the estimated steady state C max, C min and area under the curve (AUC) after a two-week dosing interval of BESREMi over a dose range of 100 mcg to 500 mcg ranged from 4.4 – 31 ng/mL, 1.4 – 12 ng/mL, and 1011 – 7809 ng×h/mL, respectively. The estimated steady state C max occurs between 2 to 5 days.
Absorption
The estimated geometric mean (CV%) of the absorption rate constant of BESREMi is 0.12 day -1 (27%) in patients with polycythemia vera.
Distribution
The estimated geometric mean (CV%) of apparent volume of distribution of BESREMi is 4.8 L (21%) in patients with polycythemia vera.
Elimination
BESREMi undergoes receptor independent degradation/excretion and receptor binding and subsequent degradation of the drug-receptor complex. The half-life and clearance of BESREMi is approximately 7 days and 1.7-2.5 L/h in patients with polycythemia vera over a dose range of 100 mcg to 500 mcg, respectively.
Specific Populations
No clinically significant differences in the pharmacokinetics of BESREMi were observed based on age, sex, body surface area, and JAK2V617F mutation.
Drug Interactions
Clinical Studies
No clinical studies evaluating the drug interaction potential of BESREMi have been conducted.
In Vitro Studies
In vitro studies indicate that BESREMi exhibited time-dependent inhibitory potential on CYP2A6. BESREMi did not inhibit CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A4 in human liver microsomes. BESREMi is not expected to induce CYP enzymes. However, interferon may influence CYP450 through modulating transcription factors and altering protein expression and/or structure. As this mechanism requires more time to exert effect, it cannot be evaluated by in vitro assays.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Ropeginterferon alfa-2b-njft has not been tested for its carcinogenic potential. Neither ropeginterferon alfa-2b-njft nor its components, interferon or methoxypolyethylene glycol, caused damage to DNA when tested in the standard battery of mutagenesis assays. Ropeginterferon alfa-2b-njft effects on fertility have not been assessed [see Use in Specific Populations (8.1, 8.2, 8.3)] .
-
14 CLINICAL STUDIES
The efficacy and safety of BESREMi were evaluated in the PEGINVERA study, a prospective, multicenter, single-arm trial of 7.5 years duration. The study included 51 adults with polycythemia vera. The mean age at baseline was 56 years (range 35-82 years) with 20 (39%) women and 31 (61%) men. All patients had the JAK2V617F mutation with 16% of subjects being newly diagnosed; 84% had known disease with a median duration of 2.2 years. One-third (33%) of patients were undergoing treatment with hydroxyurea (HU) upon study entry. At baseline, the mean ± SD hematocrit, platelets, and leukocytes were 45% ± 4.0%, 457 ×10 9/L ± 187 ×10 9/L and 11.8 × 10 9/L ± 5.2 × 10 9/L, respectively. Median spleen size was 13.2 cm with 16 (31%) having splenomegaly (defined as a longitudinal diameter of >12 cm for women and >13 cm for men). Eleven patients (22%) had a prior history of a major cardiovascular event including pulmonary embolism (6), stroke (2), myocardial infarction (2) and portal vein thrombosis (1).
In stage I, the maximum tolerated dose, defined as the highest administered dose without dose-limiting toxicities was determined to be 540 mcg. In stage II, an intra-patient dose escalation began at 150 mcg, or 100 mcg if titrating from hydroxyurea, or at the highest dose achieved in those patients enrolled during stage I. Titration with BESREMi occurred every two-weeks at doses of 225 mcg, 300 mcg, 400 mcg and 450 mcg with dose escalation stopping when hematological parameters were stabilized. For patients transitioning from hydroxyurea, the hydroxyurea dose was tapered off over the first 12 weeks of treatment to avoid toxicity. After at least one year on therapy and at a median time of 21.5 months, 28 eligible patients in the PEGINVERA study increased the dosing interval to once every 4 weeks. Because of formulation changes, the recommended starting dose, titration amounts, and maximum dose of BESREMi differ slightly from those used in the trial [see Dosage and Administration (2)] .
The median duration of treatment exposure was 61 months and 53% of patients completed at least 60 months of treatment. Thirty-six patients completed one year of treatment with eleven patients discontinuing after one year of treatment mainly due to treatment emergent adverse events. The mean dose of BESREMi was 237 mcg (± 110) during the treatment period.
The efficacy of BESREMi was evaluated in the PEGINVERA study by assessing complete hematological response (CHR) defined as hematocrit <45% and no phlebotomy in the preceding 2 months, platelets ≤400 × 10 9/L and leukocytes ≤10 × 10 9/L, normal spleen size (longitudinal diameter ≤ 12 cm for females and ≤ 13 cm for males) assessed by ultrasound and absence of thromboembolic events.
The CHR in the treated population during the treatment period was 61% (31/51) (95% CI: 46, 74). The median duration of response was 14.3 months (95% CI: 5.5, 30.1).
Among the patients in the treated population who achieved a CHR, the median time to response was 7.8 months of treatment with BESREMi. It required 1.2 years of treatment with BESREMi for 50% of patients (hydroxyurea-naïve) to achieve a CHR and 1.4 years for 50% of patients with prior hydroxyurea use to achieve a CHR.
A hematological response based only on hematocrit, platelets, and leukocytes was achieved among 80% of patients treated with BESREMi (41/51) (95% CI: 67, 90). The median duration of this response was 20.8 months (95% CI: 13.0, 43.8).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
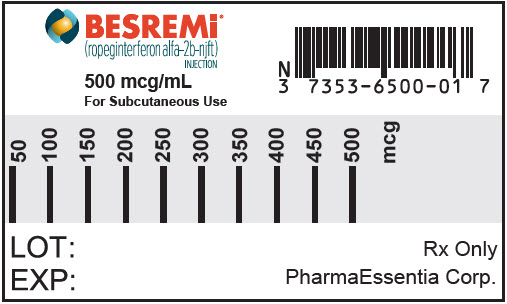
BESREMi (ropeginterferon alfa-2b-njft) injection is a sterile, preservative-free, clear and colorless to slightly yellowish solution for subcutaneous administration in a single-dose prefilled syringe. Each carton contains one 500 mcg/mL prefilled syringe with a 30 gauge, ½ inch safety hypodermic needle (NDC 73536-500-01).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Depression and Suicide
Inform patients, their caregivers, and family members that suicidal ideation and behavior, as well as new onset or worsening depression have been reported in patients treated with BESREMi. Advise them to be aware of any unusual changes in mood or behavior, new onset or worsening of depression, or the emergence of suicidal thoughts or behavior. Instruct patients, caregivers, and family members to report signs or symptoms of depression to their healthcare provider right away, but to discontinue BESREMi immediately and seek immediate medical attention if suicidal ideation or attempts occur [see Warnings and Precautions (5.1)] .
Endocrine Toxicity
Advise patients to report any signs or symptoms of diabetes or thyroid dysfunction [see Warnings and Precautions (5.2)] .
Cardiovascular Toxicity
Advise patients to report signs or symptoms of cardiovascular toxicity to their healthcare provider [see Warnings and Precautions (5.3)].
Decreased Peripheral Blood Counts
Advise patients to seek prompt medical attention if they experience weakness/fatigue, fever, easy bruising, or frequent nose bleeds [see Warnings and Precautions (5.4)].
Hypersensitivity
Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions (5.5) and Drug Interactions (7)].
Pancreatitis
Advise patients to report signs or symptoms of pancreatitis [see Warnings and Precautions (5.6)] .
Colitis
Advise patients to report signs or symptoms of colitis [see Warnings and Precautions (5.7)] .
Pulmonary Toxicity
Advise patients to report signs or symptoms of pulmonary toxicity [see Warnings and Precautions (5.8)] .
Ophthalmologic Toxicity
Advise patients to report visual changes and to have eye examinations before and during treatment [see Warnings and Precautions (5.9)] .
Hyperlipidemia
Advise patients that BESREMi may increase blood triglycerides and that they will need blood testing to monitor for this toxicity [see Warnings and Precautions (5.10)] .
Hepatotoxicity
Advise patients to report signs or symptoms of hepatic toxicity to their healthcare provider [see Warnings and Precautions (5.11) and Use in Specific Populations (8.7)] .
Renal Toxicity
Advise patients to report signs or symptoms of kidney disease [see Warnings and Precautions (5.12) and Use in Specific Populations (8.6)] .
Dental and Periodontal Toxicity
Advise patients to maintain good oral hygiene and to have regular dental examinations [see Warnings and Precautions (5.13)] .
Dermatologic Toxicity
Advise patients to seek medical attention if significant pruritus, alopecia, rash and/or other dermatological toxicities occur [see Warnings and Precautions (5.14)] .
Hazardous Occupations/Operating Machinery
Advise patients to refrain from engaging in operating heavy or potentially dangerous machinery until they know how BESREMi will affect their abilities. Advise patients who experience dizziness, somnolence and hallucinations not to drive or use heavy machinery [see Warnings and Precautions (5.15)] .
Pregnancy and Contraception
Advise women about the need to use an effective method of contraception while taking BESREMi and for at least 8 weeks after the final dose [see Use in Specific Populations (8.1, 8.3)] .
Lactation
Advise women not to breastfeed during treatment and for 8 weeks after the final dose [see Use in Specific Populations (8.2)] .
Instruction on Injection Technique
Instruct patients on proper storage, preparation and administration techniques for BESREMi. Instruct patients who are self-administering to inject the prescribed dose of BESREMi [see Dosage and Administration (2.4)] .
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Medication Guide
BESREMi ® (bez-reh-me)
ropeginterferon alfa-2b-njft
injection, for subcutaneous useThis Medication Guide has been approved by the U.S. Food and Drug Administration. Issued: 11/2021 What is the most important information I should know about BESREMi? BESREMi can cause serious side effects that: - may cause death, or
- may worsen certain serious diseases that you may already have
Tell your healthcare provider right away if you have any of the symptoms listed below during treatment with BESREMi. If symptoms get worse, or become severe and continue, your healthcare provider may tell you to stop taking BESREMi permanently. These symptoms may go away in some people after they stop taking BESREMi. Mental health problems, including suicide. BESREMi may cause you to develop mood or behavior problems that may get worse during treatment with BESREMi or after your last dose, including: - irritability (getting upset easily)
- restlessness and agitation
- confusion
- depression (feeling low, feeling bad about yourself or feeling hopeless)
- unusually grand ideas
- acting aggressive
- acting impulsively
- thoughts of hurting yourself or others, or thoughts of suicide
If you develop any of these symptoms, stop using BESREMi right away. You, your caregiver, or family member should call your healthcare provider right away. Your healthcare provider should carefully monitor you during treatment with BESREMi. New or worsening autoimmune problems. BESREMi may cause autoimmune problems (a condition where the body's immune cells attack other cells or organs in the body), including thyroid problems, increased blood sugar (hyperglycemia), and type I diabetes. In some people who already have an autoimmune problem, it may get worse during your treatment with BESREMi. Tell your healthcare provider if you have any of the following symptoms: tiredness, urinating often, or if you are very thirsty. Heart problems. BESREMI may cause heart problems, including problems with your heart muscle (cardiomyopathy), heart attack, abnormal heart rhythm (atrial fibrillation), and decreased blood flow to your heart. You should not use BESREMi if you: have high blood pressure that is not controlled, congestive heart failure, a serious abnormal heart rhythm, narrowing of the arteries to your heart, certain types of chest pain (angina), or a recent stroke or heart attack. If you have a heart problem before you start using BESREMi, your healthcare provider should monitor you closely during treatment with BESREMi. Call your healthcare provider right away if you get any of the symptoms listed above during treatment with BESREMi. Before and during treatment with BESREMi you will need to see your healthcare provider regularly and have blood tests to monitor your polycythemia vera, and to check for side effects. BESREMi can cause serious side effects. Some of these side effects may cause death. Tell your healthcare provider right away if you have any of the symptoms listed above during treatment with BESREMi. For more information about side effects, see " What are the possible side effects of BESREMi?" What is BESREMi? BESREMi is a prescription medicine that is used to treat adults with polycythemia vera. It is not known if BESREMi is safe and effective in children. Who should not use BESREMi? Do not use BESREMi if you: - have or had severe mental health problems, especially severe depression, thoughts of suicide, or attempted suicide
- have or had a serious or untreated autoimmune disease
- have had a serious allergic reaction to another interferon product or to any of the ingredients in BESREMi. Symptoms of a serious allergic reaction to alpha-interferon may include itching, swelling of your face, tongue, throat, trouble breathing, feeling dizzy or faint, and chest pain. See the end of this Medication Guide for a complete list of ingredients in BESREMi. Ask your healthcare provider if you are not sure.
- have certain types of liver problems
- have received a transplant and take immunosuppressive medicines
Talk to your healthcare provider before taking BESREMi if you have any of these conditions. Before using BESREMi, tell your healthcare provider about all of your medical conditions, including if you: - are being treated for a mental illness or had treatment in the past for any mental illness, including depression and have had thoughts of hurting yourself or others.
- have type 1 diabetes
- have or ever had any problems with your heart, including heart attack or high blood pressure
- have or ever had bleeding problems or a blood clot
- have or ever had low blood cell counts
- have a condition that suppresses your immune system, such as certain cancers
- have hepatitis B infection
- have HIV infection
- have kidney problems
- have liver problems
- are pregnant or plan to become pregnant. BESREMi may harm your unborn baby and may cause loss of your pregnancy (miscarriage).
- Before you start using BESREMi your healthcare provider should do a pregnancy test.
- You should use effective birth control during treatment and for at least 8 weeks after your final dose of BESREMi. Talk to your healthcare provider about birth control choices for you during treatment with BESREMi.
- BESREMi can affect your menstrual cycles and may cause your menstrual periods to stop.
- Tell your healthcare provider if you become pregnant during treatment with BESREMi.
- are breastfeeding or plan to breastfeed. It is not known if BESREMi passes into your breast milk. You should not breastfeed during treatment and for 8 weeks after your final dose of BESREMi.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. BESREMi and certain other medicines may affect each other and cause side effects. Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider and pharmacist when you get a new medicine. How should I use BESREMi? - See the Instructions for Use that comes with BESREMi for detailed instructions on how to prepare and inject a dose of BESREMi.
- Use BESREMi exactly as your healthcare provider tells you to. Your healthcare provider will tell you how much BESREMi to inject and when to inject it. Do not inject more than your prescribed dose.
- BESREMi is given as an injection under your skin (subcutaneous injection). Your healthcare provider should show you how to prepare and measure your dose of BESREMi, and how to inject yourself before you use BESREMi for the first time.
- You should not inject BESREMi until your healthcare provider has shown you how to use BESREMi the right way. Your healthcare provider will prescribe the amount of BESREMi that is right for you.
- Do not inject more than 1 dose of BESREMi every 2 weeks without talking to your healthcare provider. Do not re-use the single-dose prefilled syringe.
- Your healthcare provider should do blood tests before you start BESREMi, and regularly during treatment to monitor your polycythemia vera, and to check you for side effects.
What should I avoid during treatment with BESREMi? - BESREMi may cause neurologic symptoms including dizziness, sleepiness, and hallucinations. Avoid driving or using machinery if you develop any of these neurologic symptoms during treatment with BESREMi.
What are the possible side effects of BESREMi? BESREMi can cause serious side effects including: - See " What is the most important information I should know about BESREMi?"
- Decreased blood cell counts. Decreased blood cell counts are common with BESREMi and can sometimes also be severe, especially decreased platelets or white blood cells. Your red blood cells may also be decreased. Your healthcare provider should check your blood cell counts before you start and during treatment with BESREMi. If your blood cell counts are low you can develop anemia, infections or have problems with bleeding or bruising.
Call your healthcare provider right away if you develop any of the following symptoms: - weakness and tiredness
- bruising easily
- you have nose bleeds often
- fever
- chills
- burning and painful urination
- urinating often
- coughing up yellow or pink mucus (phlegm)
- Serious allergic reactions and skin reactions. BESREMi can cause serious, sudden allergic reactions.
Get medical help right away if you get any of the following symptoms: - skin rash or hives
- itching swelling of your face, eyes, lips tongue, or throat
- trouble breathing
- chest pain
- feeling faint
- Eye problems. BESREMi can cause severe eye problems with your retinas that can lead to vision loss or blindness. You should have an eye exam before and during treatment with BESREMi if you have diabetes or high blood pressure and also have retinal problems. Your healthcare provider may stop BESREMi if you develop new or worse eye problems during treatment with BESREMi.
- Liver problems. BESREMi can cause increases in liver enzymes and liver damage. Your healthcare provider should do blood tests to monitor your liver enzymes and liver function before you start and during treatment with BESREMi.
- Kidney problems. Your healthcare provider will do blood tests to check your kidney function before starting and during treatment with BESREMi. Tell your healthcare provider right away if you develop any symptoms of a kidney problem, including:
- changes in the amount or color of your urine
- blood in your urine
- swelling in your ankles
- loss of appetite
Your healthcare provider may stop BESREMi if you develop severe kidney problems. - Tooth and gum (periodontal) problems. BESREMi can cause tooth and gum problems which can lead to tooth loss. BESREMi can also cause problems with dry mouth that can damage your teeth and the lining of the mouth during long-term treatment with BESREMi. It is important for you to brush your teeth well, two times each day and have regular dental examinations during treatment with BESREMI.
- Skin problems. BESREMi can cause skin problems. Signs and symptoms of a skin problem with BESREMi include:
- itching
- hair loss
- rash
- redness
- psoriasis
- acne
- thickening of the skin
- excessive sweating
Call your healthcare provider if you develop a rash that is bothersome or covers a large skin area. The most common side effects of BESREMi include: - flu like symptoms including: tiredness, weakness, fever, chills, muscle aches, and joint pain
- itching
- sore throat.
These are not all of the possible side effects of BESREMi. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store BESREMi? - Store BESREMi in the refrigerator between 36° F to 46° F (2° C to 8° C).
- Keep BESREMi away from heat.
- Do not freeze BESREMi.
- Keep the BESREMi pre-filled syringe in the outer carton to protect it from light.
Keep BESREMi and all medicines out of the reach of children. General information about the safe and effective use in BESREMi? Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BESREMi for a condition for which it has not been prescribed. Do not give BESREMi to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about BESREMi that is written for health professionals. What are the ingredients in BESREMi? Active ingredient: ropeginterferon alfa-2b-njft Inactive ingredients: benzyl alcohol, glacial acetic acid, polysorbate 80, sodium acetate, sodium chloride, Water for Injection. Manufactured by: PharmaEssentia Corporation 2F-5 No. 3 YuanQu Street Nangang Dist. Taipei, Taiwan
U.S. License number: 2155
Distributed by: PharmaEssentia USA Corporation, 35 Corporate Dr, Suite 325, Burlington, MA 01803, USA
BESREMi is a trademark/registered trademark of PharmaEssentia
Copyright 2021 PharmaEssentia
For more information, go to www.BESREMi.com or call 1-800-999-2449 -
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
BESREMi [bez-reh-me]
(ropeginterferon alfa-2b-njft)
injection, for subcutaneous use
Single-dose prefilled syringeThis "Instructions for Use" has been approved by the U.S. Food and Drug Administration. Issued: November 2021 This "Instructions for Use" contains information on how to prepare and inject BESREMi under your skin (subcutaneous injection) using the single-dose prefilled syringe. Guide to Prefilled syringe and Needle Parts (Figure A) 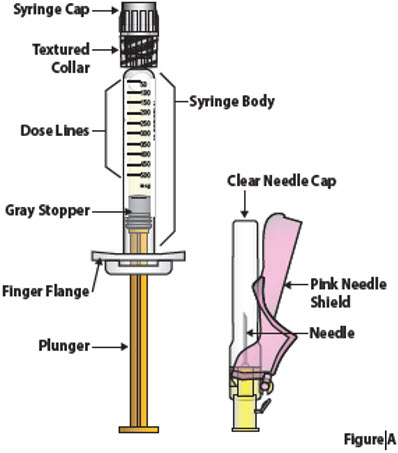
Storing BESREMi Store the BESREMi carton in the refrigerator between 36°F to 46°F (2°C to 8°C) (Figure B). - Keep your BESREMi prefilled syringes in their original carton (Figure B) while stored.
- Do not freeze the prefilled syringes.
- Do not use a prefilled syringe that has been frozen or left in direct sunlight.
- Keep BESREMi prefilled syringes, needles, and all medicines out of the reach of children.
Important information you need to know before injecting BESREMi Read this Instructions for Use before using your single-dose BESREMi prefilled syringe for the first time and each time you get a new prescription. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment. Ask your healthcare provider about the right way to prepare and give your BESREMi injection. - Your healthcare provider will tell you the prescribed dose that you should take and the right amount of BESREMi to measure in the prefilled syringe for your dose. Each time you inject, be sure that you know the prescribed dose of BESREMi to inject. Your dose may change over time.
- BESREMi is for subcutaneous (under the skin) injection only.
- BESREMi is for one-time use only. Do not reuse your prefilled syringe or needle.
- Do not use a prefilled syringe or needle that is damaged or broken. Contact your healthcare provider for a replacement prefilled syringe or additional needles.
- Inject BESREMi into the top of the thighs or lower stomach-area just under the skin. Do not inject BESREMi into any other area of the body.
- Throw away (dispose of) the BESREMi prefilled syringe with needle attached right away after use, even if there is medicine left in the prefilled syringe. See Step 10 in the section " Dispose of used prefilled syringes and needles."
Gather and check supplies 1. Prepare BESREMi Prefilled Syringe 1.1. Take the BESREMi carton out of the refrigerator (Figure E). 
1.2. Check the expiration date ("EXP") on the top panel of the carton to make sure it has not passed (Figure F).
Do not use the prefilled syringe if the expiration date has passed.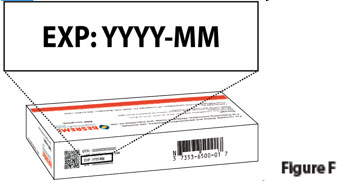
1.3. Let carton containing the BESREMi prefilled syringe sit on a clean work surface for 15 to 30 minutes to allow it to come to room temperature (Figure G).
Do not warm the prefilled syringe any other way.
2. Gather supplies for injection 2.1. After allowing the prefilled syringe to come to room temperature for 15 to 30 minutes inside the carton, gather the following additional supplies.
Alcohol Swab (Figure H).
FDA-cleared Sharps Disposal Container (Figure I)
A paper towel, sink, or trash can to minimize mess during dose adjustment (Figure J).
Optional Items: Gauze or Cotton Ball and a Small Adhesive Bandage (Figure K).
3. Wash hands and remove syringe from tray 3.1. Wash your hands with soap and water, then dry your hands (Figure L). 
3.2. Open the carton and remove the clear plastic tray that holds the BESREMi prefilled syringe and needle package (Figure M). 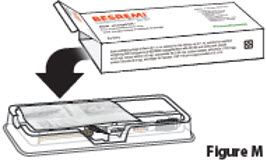
3.3. Remove the needle package and BESREMi prefilled syringe from the plastic tray. Hold the prefilled syringe by the middle of the syringe body during removal (Figure N). 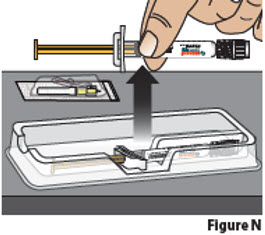
4. Check the liquid medicine in the BESREMi prefilled syringe 4.1. Check the liquid medicine in the prefilled syringe (Figure O). The liquid should be clear and colorless to slightly yellow, and should not have particles.
Do not use the prefilled syringe if the liquid is cloudy, discolored, or contains particles. Contact your healthcare provider or pharmacist.4.2. Check the syringe to see if it is damaged or broken (Figure O).
Do not use if it shows any signs of damage or breakage. Contact your healthcare provider or pharmacist.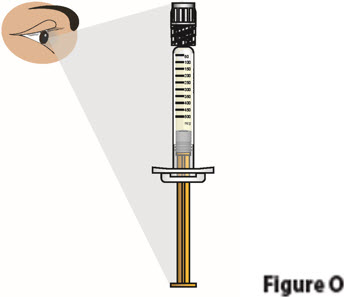
Prepare syringe for injection 5. Attach the needle to the BESREMi prefilled syringe 5.1. Carefully open the needle package, remove the needle, and set it aside (Figure P).
Throw away the packaging into household trash.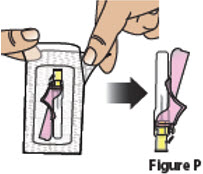
5.2. Hold the prefilled syringe as shown. Remove the prefilled syringe cap by unscrewing it counter-clockwise (Figure Q).
Throw away the syringe cap into household trash.
Do not allow the tip of the prefilled syringe to touch anything.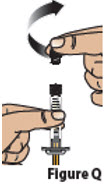
5.3. Attach the needle to the prefilled syringe by firmly pushing it into the collar of the syringe and then screwing (turn clock-wise) it on until it feels securely attached (Figure R).
The needle should now be assembled to the prefilled syringe (Figure S).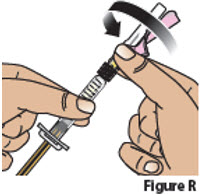

6. Choose and clean injection site 6.1. Choose one of the following injection sites (Figure T): - Lower stomach (abdomen) area, at least 2 inches away from the belly button,
- Top of thighs.
Do not inject into skin that is irritated, red, bruised, infected, or scarred.
BESREMi is for subcutaneous (under the skin) injection only.
Rotate (change) the injection site for each injection.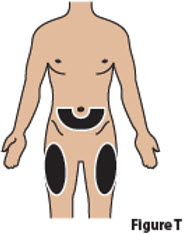
6.2. Clean the chosen injection site with an alcohol swab and let it air dry (Figure U).
Do not blow on or touch the injection site after it has been cleaned.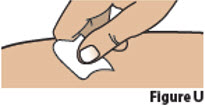
7. Uncap needle and move air bubbles to top 7.1. Pull the pink needle shield back (Figure V).
Note: The pink needle shield will be used after the injection to cover the needle and protect you from needle-stick injuries.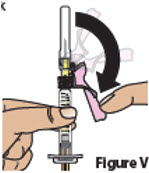
7.2. Hold the syringe from the syringe body.
Remove the clear needle cap by pulling it straight off (Figure W). Throw away the needle cap into household trash.
Do not recap needle.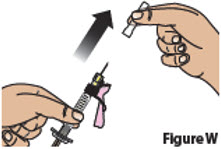
7.3. Hold the prefilled syringe with the needle pointing up.
Tap on the body of the prefilled syringe to move any air bubbles to the top (Figure X).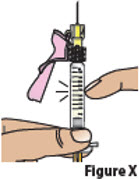
8. Set your dose 8.1. Check your prescription to identify your prescribed dose (Figure Y). Depending on your prescribed dose, you may have to adjust the dose in the syringe by getting rid of (discarding) some medicine from the prefilled syringe before you inject the medicine. 
8.2. To set your dose follow the 4 steps below: - Hold the prefilled syringe at eye level with the needle pointing straight up over a paper towel, sink, or trash can.
- Check that you can see the dose lines and number markings on the prefilled syringe.
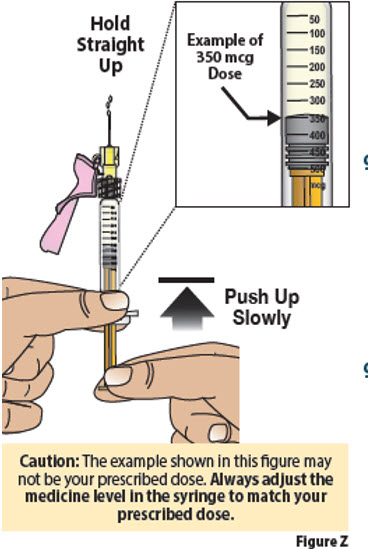
- Pinch the end of the plunger as shown (Figure Z).
-
Slowly push up on the plunger to remove liquid medicine until the top edge of the gray stopper lines up with the marking for
your prescribed dose (Figure Z).
Keep holding straight up as you set the dose.
Important: If you accidentally remove too much liquid medicine, do not inject. Contact your healthcare provider or pharmacist.
Inject BESREMi 9. Give Injection 9.1. Pinch the chosen injection site (Figure AA). 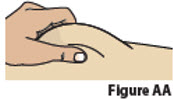
9.2. While pinching the skin, insert the needle at a 45 to 90 degree angle into the pinched skin (Figure AB).
Then release the pinched skin.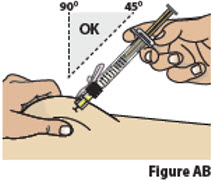
9.3. Inject the medicine by slowly pressing down on the plunger all the way until it stops (Figure AC). 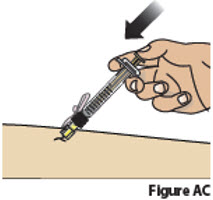
9.4. After all the liquid medicine is injected, remove the needle from the skin (Figure AD). 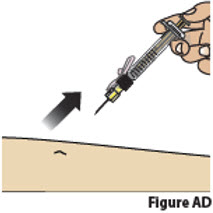
9.5 Cover needle
Carefully push the pink needle shield over the needle until it snaps into place and covers the needle (Figure AE). This helps prevent needle-stick injuries.
Do not recap the needle using the needle cap. Only use the pink needle shield to cover the needle.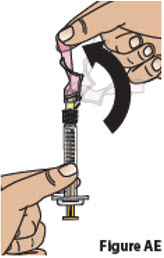
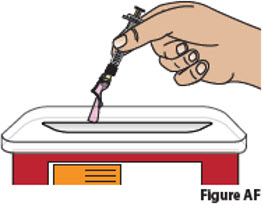
Do not reuse the prefilled syringe and needle. Disposing of used prefilled syringes and needles 10. Dispose of used prefilled syringes and needles. - Put your used prefilled syringes and needles in a FDA-cleared sharps disposal container right away after use (Figure AF).
Do not throw away (dispose of) loose prefilled syringes and needles in the household trash.
- If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
- When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes. For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA's website at: http://www.fda.gov/safesharpsdisposal.
- Do not dispose of your sharps disposal container in your household trash unless your community guidelines permit this. Do not recycle your used sharps disposal container.
- Always keep the sharps disposal container out of the reach of children.
11 Check injection site. 11.1 If there is a small amount of blood or liquid at the injection site, press a gauze or cotton ball over the injection site until the bleeding stops (Figure AG). 11.2 Do not rub the injection site. If needed, you may apply a small adhesive bandage. 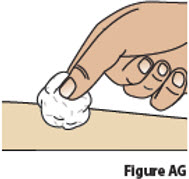
Additional information. For additional information about BESREMi and a video demonstration on how to use BESREMi, go to:
www.BESREMi.comManufactured by:
PharmaEssentia Corporation
2F-5 No. 3 YuanQu Street
Nangang Dist. Taipei, Taiwan
U.S. License number: 2155Distributed by:
PharmaEssentia Corporation
35 Corporate Drive, Suite 325, Burlington, MA 01803, USABESREMi is a trademark/registered trademark of PharmaEssentia
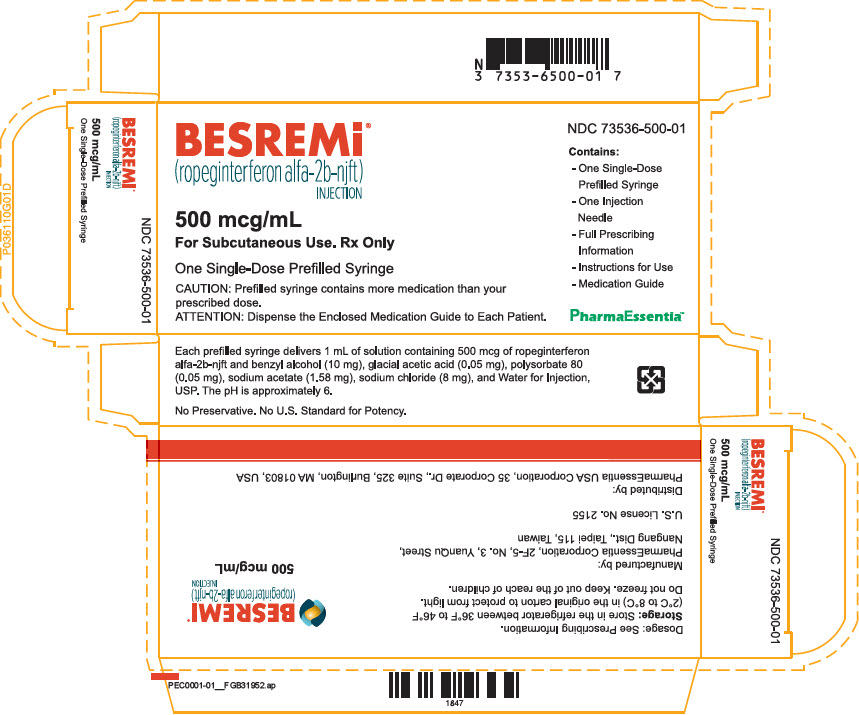
Copyright 2021 PharmaEssentia - PRINCIPAL DISPLAY PANEL - 500 mcg/mL Syringe Label
-
PRINCIPAL DISPLAY PANEL - 500 mcg/mL Syringe Carton
BESREMi ®
(ropeginterferon alfa-2b-njft)
INJECTION500 mcg/mL
For Subcutaneous Use. Rx OnlyOne Single-Dose Prefilled Syringe
CAUTION: Prefilled syringe contains more medication than your
prescribed dose.ATTENTION: Dispense the Enclosed Medication Guide to Each Patient.
NDC 73536-500-01
Contains:
- One Single-Dose
Prefilled Syringe - One Injection
Needle - Full Prescribing
Information - Instructions for Use
- Medication Guide
PharmaEssentia™
- One Single-Dose
-
INGREDIENTS AND APPEARANCE
BESREMI
ropeginterferon alfa-2b injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73536-500 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ROPEGINTERFERON ALFA-2B (UNII: 981TME683S) (ROPEGINTERFERON ALFA-2B - UNII:981TME683S) ROPEGINTERFERON ALFA-2B 500 ug in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 10 mg in 1 mL ACETIC ACID (UNII: Q40Q9N063P) POLYSORBATE 80 (UNII: 6OZP39ZG8H) SODIUM ACETATE (UNII: 4550K0SC9B) SODIUM CHLORIDE (UNII: 451W47IQ8X) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73536-500-01 1 in 1 CARTON 11/12/2021 1 1 mL in 1 SYRINGE, GLASS; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA761166 11/12/2021 Labeler - PharmaEssentia USA (080514277) Establishment Name Address ID/FEI Business Operations PharmaEssentia Corporation, Taichung Plant 658873411 api manufacture(73536-500) Establishment Name Address ID/FEI Business Operations PYRAMID Laboratories, Inc. 783409162 analysis(73536-500) , manufacture(73536-500)