Label: DUKAL ALCOHOL PREP PAD- isopropyl alcohol swab
- NDC Code(s): 65517-0001-1
- Packager: Dukal LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated April 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
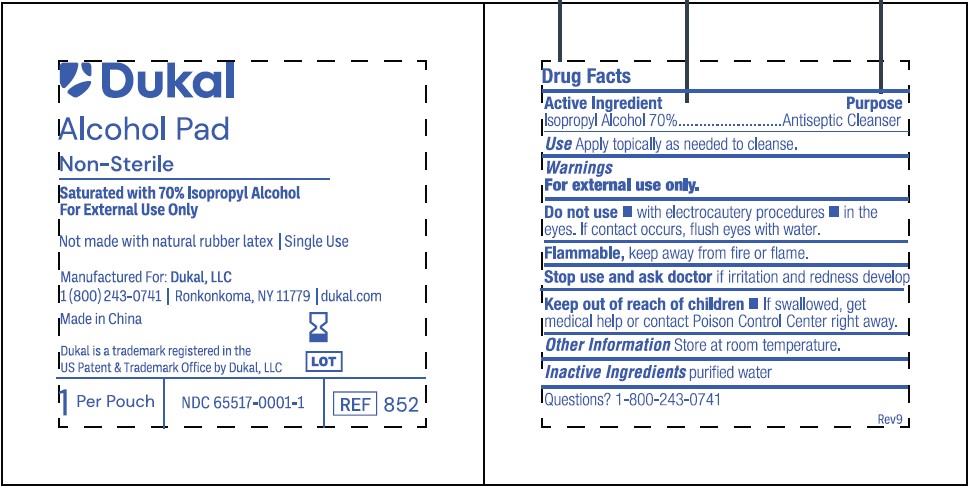
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
-
PRINCIPAL DISPLAY PANEL
Dukal
Alcohol Pad
Non-Sterile
Saturated with 70% Isopropyl Alcohol
For External Use Only
Not made with natural rubber latex / Single Use
Manufactured For: Dukal, LLC
1(800)243-0741 /Ronkonkoma, NY 11779 / dukal.com
Made in China
Dukal is a trademark registered in the
US Patent & Trademark Office by Dukal, LLC
1 Per Pouch / NDC 65517-0001-1 / REF 852
-
INGREDIENTS AND APPEARANCE
DUKAL ALCOHOL PREP PAD
isopropyl alcohol swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65517-0001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-0001-1 0.4 mL in 1 POUCH; Type 0: Not a Combination Product 05/01/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 05/01/2010 Labeler - Dukal LLC (791014871)

