Label: EMORY JOHNS CREEK AMENITY- alcohol and sodium monofluorophospate kit
- NDC Code(s): 42555-060-45, 59448-004-01, 59448-700-00
- Packager: ASP Global, LLc
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 15, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
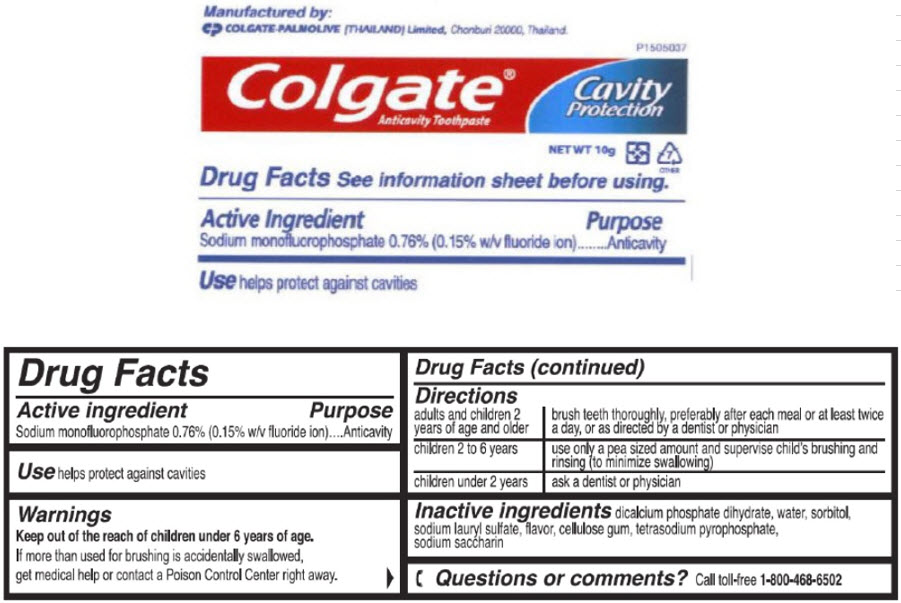
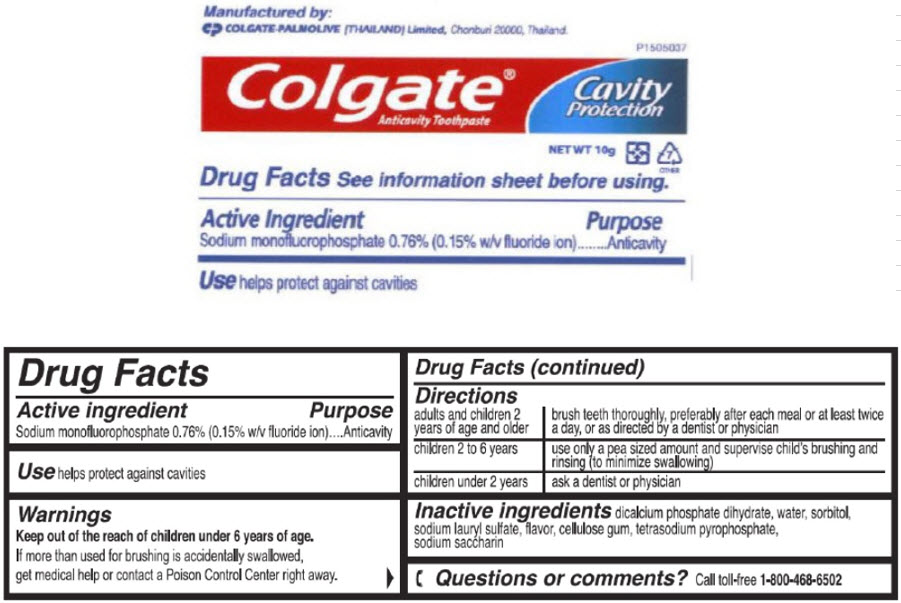
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
adults and children 2 years of age and older brush teeth thoroughly, preferably after each meal or at least twice a day, or as directed by a dentist or physician children 2 to 6 years use only a pea sized amount and supervise child's brushing and rinsing (to minimize swallowing) children under 2 years ask a dentist or physician - Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 53 mL Bottle Label
- PRINCIPAL DISPLAY PANEL - 10 g Tube Label
-
PRINCIPAL DISPLAY PANEL - Kit Label
EMORY
JOHNS CREEK
HOSPITALItem #: EJCKIT01
Description: AMENITY KIT,
EMORY JOHNS CREEK
PO #:
Lot #: XMMDDFC
Exp: YYYY-MM-DD
Qty: 30 KIT/CS
Carton #: XXX of XXXX
Net Wt.: XX KG
Gross Wt.: XX KG
Cubic Dimensions: 54.0 x 19.0 x 27.0 CMAssembled in China
Components Made in China:
- Alcohol and Fragrance Free Hand Sanitizer
- Lip Balm Ball
- Toothbrush
- Sleep Mask
- Ear Plugs
- Body Lotion
- Deodorant Stick
- Kit Case
Component Made in Thailand:
- Toothpaste
EMORY
JOHNS CREEK
HOSPITALOn behalf of the entire Emory Johns Creek Hospital family,
we thank you for trusting us with your care. Our goal is to
provide all our patients with a nurturing and healing
environment, and we hope this kit adds to your comfort
during your stay. Please let us know what we can do to
make your stay more comfortable.Contents/Origins:
1.9 fl. oz. Hand Sanitizer,
0.25 oz. net wt. Lip Balm Ball,
2 fl. oz. Body Lotion,
0.5 oz. net wt. Deodorant Stick,
Toothbrush, Sleep Mask, Ear Plugs, and Kit Case:
Made in China
Toothpaste: Made in ThailandLot #: XMMDDFC
Exp: YYYY-MM-DDDistributed by:
ASP Global, LLC
7800 Third Flag Parkway, Austell, GA 30168, USAREV 02
EMORY
JOHNS CREEK
HOSPITAL
-
INGREDIENTS AND APPEARANCE
EMORY JOHNS CREEK AMENITY
alcohol and sodium monofluorophospate kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59448-700 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59448-700-00 30 in 1 BOX 07/27/2020 1 1 in 1 BAG Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 BOTTLE, PLASTIC 53 mL Part 2 1 TUBE 10 g Part 3 1 TUBE 59 mL Part 4 1 TUBE 4.25 g Part 5 1 BOTTLE, WITH APPLICATOR 14 g Part 1 of 5 INSTANT HAND ANTISEPTIC
alcohol gelProduct Information Item Code (Source) NDC:59448-004 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Alcohol (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) Alcohol 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Aloe Vera Leaf (UNII: ZY81Z83H0X) Glycerin (UNII: PDC6A3C0OX) Propylene Glycol (UNII: 6DC9Q167V3) Carbomer Interpolymer Type A (Allyl Sucrose Crosslinked) (UNII: 59TL3WG5CO) Trolamine (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59448-004-01 53 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part333E 10/15/2019 Part 2 of 5 COLGATE ANTICAVITY
sodium monofluorophosphate paste, dentifriceProduct Information Item Code (Source) NDC:42555-060 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Monofluorophosphate (UNII: C810JCZ56Q) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 7.6 mg in 1 g Inactive Ingredients Ingredient Name Strength DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) Water (UNII: 059QF0KO0R) Sorbitol (UNII: 506T60A25R) Sodium Lauryl Sulfate (UNII: 368GB5141J) Sodium Pyrophosphate (UNII: O352864B8Z) Saccharin Sodium (UNII: SB8ZUX40TY) Product Characteristics Color WHITE Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:42555-060-45 10 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part355 06/04/2009 Part 3 of 5 GILCHRIST AND SOAMES BODY BUTTER
cleansing (cold creams, cleansing lotions, liquids, and pads)Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) INGR CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) INGR GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) INGR CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) INGR ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) INGR BENZYL ALCOHOL (UNII: LKG8494WBH) INGR PEG-100 STEARATE (UNII: YD01N1999R) INGR CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) INGR DEHYDROACETIC ACID (UNII: 2KAG279R6R) INGR SODIUM HYDROXIDE (UNII: 55X04QC32I) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 59 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 07/01/2013 Part 4 of 5 EMORY JOHNS CREEK LIP BALM
lipstickProduct Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR ETHYLHEXYL PALMITATE (UNII: 2865993309) INGR MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) INGR POLYISOBUTYLENE (2300 MW) (UNII: DSQ2V1DD1K) INGR YELLOW WAX (UNII: 2ZA36H0S2V) INGR HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) INGR SHEANUT OIL (UNII: O88E196QRF) INGR .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) Product Characteristics Color YELLOW Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 4.25 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 06/13/2018 Part 5 of 5 GILCHRIST AND SOAMES DEODORANT
deodorants (underarm)Product Information Other Ingredients Ingredient Kind Ingredient Name Quantity INGR Water (UNII: 059QF0KO0R) INGR GLYCERIN (UNII: PDC6A3C0OX) INGR Stearic Acid (UNII: 4ELV7Z65AP) INGR Polysorbate 20 (UNII: 7T1F30V5YH) INGR Sodium Hydroxide (UNII: 55X04QC32I) INGR WHITE WAX (UNII: 7G1J5DA97F) INGR Starch, Rice (UNII: 4DGK8B7I3S) INGR Lavender Oil (UNII: ZBP1YXW0H8) INGR Tannic Acid (UNII: 28F9E0DJY6) INGR Sodium Lactate (UNII: TU7HW0W0QT) INGR Butylene Glycol (UNII: 3XUS85K0RA) INGR PHENOXYETHANOL (UNII: HIE492ZZ3T) INGR Benzyl Alcohol (UNII: LKG8494WBH) INGR Coumarin (UNII: A4VZ22K1WT) INGR LINALOOL, (+/-)- (UNII: D81QY6I88E) INGR LIMONENE, (+)- (UNII: GFD7C86Q1W) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 14 g in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date COSMETIC 10/24/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333E 07/27/2020 Labeler - ASP Global, LLc (080361159) Establishment Name Address ID/FEI Business Operations Shengzhou Kingbird Travel Products Co., Ltd. 560219293 PACK(59448-700) , LABEL(59448-700) Establishment Name Address ID/FEI Business Operations Colgate-Palmolive (Thailand) LTD 672044552 MANUFACTURE(59448-700) Establishment Name Address ID/FEI Business Operations Nantong Health & Beyond Hygienic Products Inc. 421280161 MANUFACTURE(59448-700)