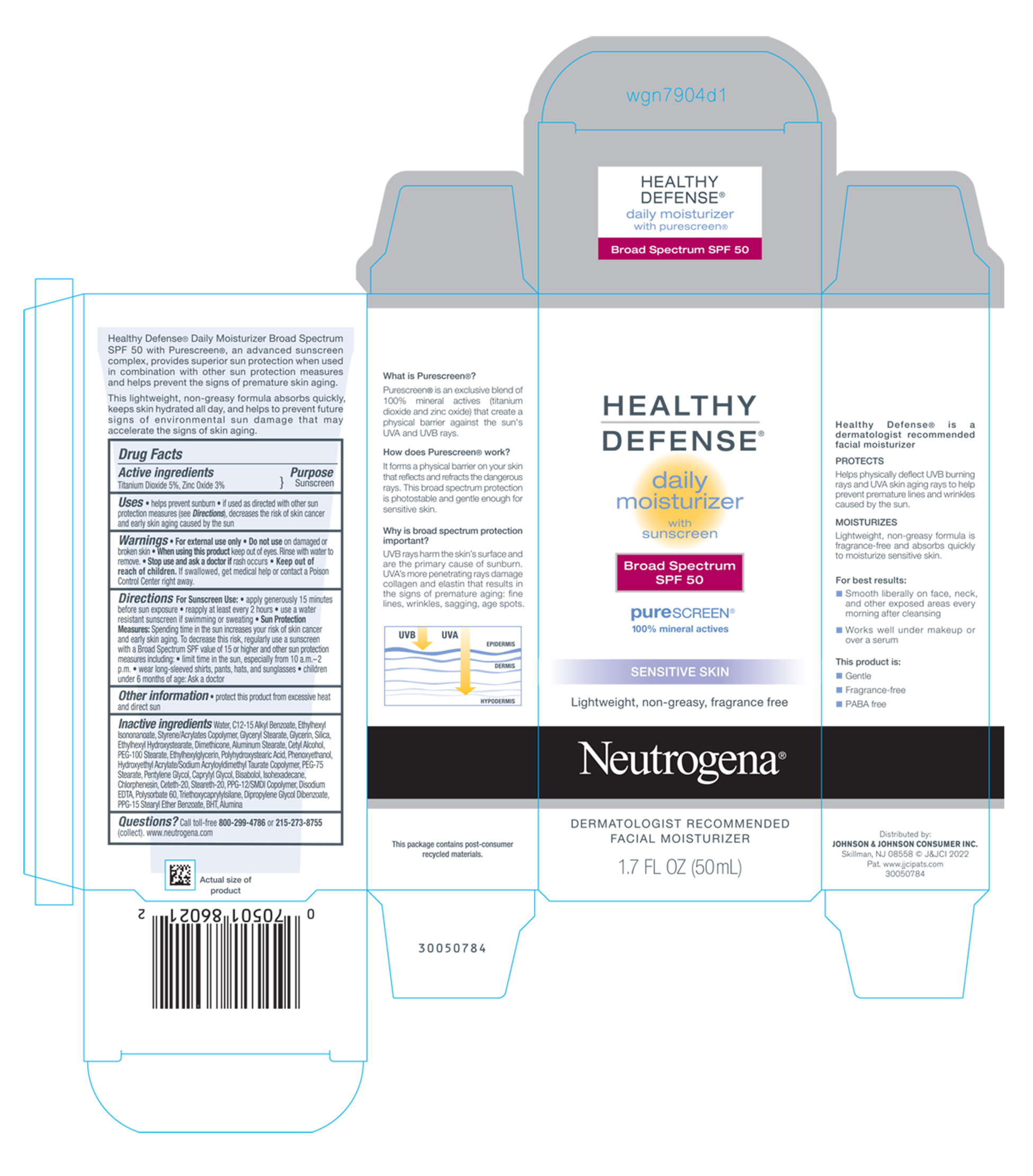
Label: NEUTROGENA HEALTHY DEFENSE DAILY MOISTURIZER WITH SUNSCREEN BROAD SPECTRUM SPF 50- titanium dioxide and zinc oxide lotion
- NDC Code(s): 69968-0515-1
- Packager: Johnson & Johnson Consumer Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 7, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions For Sunscreen Use
- apply generously 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor
- Other information
-
Inactive ingredients
water, C12-15 Alkyl Benzoate, Ethylhexyl Isononanoate, Styrene/Acrylates Copolymer, Glyceryl Stearate, Glycerin, Silica, Ethylhexyl Hydroxystearate, Dimethicone, Aluminum Stearate, Cetyl Alcohol, PEG-100 Stearate, Ethylhexylglycerin, Polyhydroxystearic Acid, Phenoxyethanol, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, PEG-75 Stearate, Pentylene Glycol, Caprylyl Glycol, Bisabolol, Isohexadecane, Chlorphenesin, Ceteth-20, Steareth-20, PPG-12/SMDI Copolymer, Disodium EDTA, Polysorbate 60, Triethoxycaprylylsilane, Dipropylene Glycol Dibenzoate, PPG-15 Stearyl Ether Benzoate, BHT, Alumina
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
NEUTROGENA HEALTHY DEFENSE DAILY MOISTURIZER WITH SUNSCREEN BROAD SPECTRUM SPF 50
titanium dioxide and zinc oxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69968-0515 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 50 mg in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 30 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) ETHYLHEXYL ISONONANOATE (UNII: I6KB4GE3K4) BUTYL METHACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID/STYRENE CROSSPOLYMER (UNII: V5RS026Q0H) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) GLYCERIN (UNII: PDC6A3C0OX) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ETHYLHEXYL HYDROXYSTEARATE (UNII: B7I80BVV5E) DIMETHICONE (UNII: 92RU3N3Y1O) ALUMINUM STEARATE (UNII: U6XF9NP8HM) CETYL ALCOHOL (UNII: 936JST6JCN) PEG-100 STEARATE (UNII: YD01N1999R) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) PEG-75 STEARATE (UNII: OT38R0N74H) PENTYLENE GLYCOL (UNII: 50C1307PZG) CAPRYLYL GLYCOL (UNII: 00YIU5438U) LEVOMENOL (UNII: 24WE03BX2T) ISOHEXADECANE (UNII: 918X1OUF1E) CHLORPHENESIN (UNII: I670DAL4SZ) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) PPG-12/SMDI COPOLYMER (UNII: 1BK9DDD24E) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) POLYSORBATE 60 (UNII: CAL22UVI4M) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) DIPROPYLENE GLYCOL DIBENZOATE (UNII: 6OA5ZDY41O) PPG-15 STEARYL ETHER BENZOATE (UNII: 80D2J6361M) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALUMINUM OXIDE (UNII: LMI26O6933) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69968-0515-1 1 in 1 CARTON 12/01/2009 07/20/2024 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 12/01/2009 07/20/2024 Labeler - Johnson & Johnson Consumer Inc. (118772437)