Label: XL-3 DAY TIME- acetaminophen, dextromethorphan hbr, phenylephrine hcl liquid
- NDC Code(s): 76281-304-28, 76281-306-25
- Packager: AptaPharma Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 30, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients (in each 15 mL, 1 tablespoon)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if • adult takes more than 4,000 mg of acetaminophen in 24 hours • child takes more than 5 doses in 24 hours • taken with other drugs containing acetaminophen • adult has 3 or more alcoholic drinks every day while using this product.
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms
may include: • skin reddening • blisters • rash. If a skin reaction occurs,
stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, lasts for more than 2 days, is
accompanied or followed by fever, headache, rash, nausea, or vomiting,
consult a doctor promptly. -
Do not use
• with any other products containing acetaminophen (prescription or nonprescription). lf you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist • for more than10 days for pain unless directed by a doctor • for more than 3 days for fever unless directed by a doctor • if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drugs. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use
- When using this product
-
Stop use and ask a doctor if
• you get nervous, dizzy or sleepless
• symptoms get worse or last more than 5 days (children) or 7days (adults)
• fever gets worse or lasts more than 3 days • redness or swelling is
present • new symptoms occur • cough comes back, or occurs with
rash or headache that lasts. These could be signs of a serious condition. - PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
-
Overdose warning:
Taking more than the recommended dose
(overdose) may cause serious health problems including liver damage.
In case of accidental overdose, get medical help or contact a Poison
Control Center (1-800-222-1222) right away.
Prompt medical attention is critical for adults as well as children even if
you do not notice any signs or symptoms. -
DOSAGE & ADMINISTRATION
Directions • take only as directed - see Overdose warning
• use dose cup or tablespoon • do not exceed 4 doses per 24 hours
• if taking Day Time during the day and Night Time at night, limit total to
4 doses per 24 hours
adults & children 12 years and over 30 mL (2 TBSP) every 4 hours
children 6 to under 12 years 15mL (1TBSP) every 4 hours
children 4 to under 6 years ask a doctor
children under 4 years do not use
- Other information
- Inactive ingredients
- Questions or Comments?
-
Principal Display Package
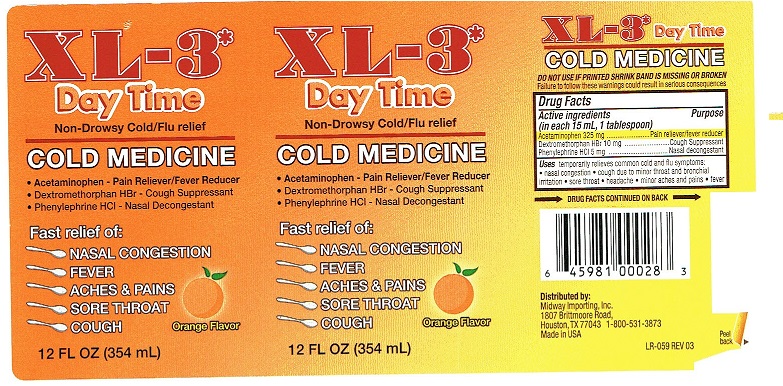

XL-3*
Day Time
Non-Drowsy Cold/Flu reliefCOLD MEDICINE
• Acetaminophen - Pain Reliever/Fever Reducer
• Dextromethorphan HBr - Cough Suppressant
• Phenylephrine HCI - Nasal DecongestantFast Relief of:
NASAL CONGESTION
FEVER
ACHES & PAINS
SORE THROAT
COUGH12 FL OZ (354 mL) Orange Flavor
XL-3*
Day Time
Non-Drowsy Cold/Flu reliefCOLD MEDICINE
• Acetaminophen - Pain Reliever/Fever Reducer
• Dextromethorphan HBr - Cough Suppressant
• Phenylephrine HCI - Nasal DecongestantFast Relief of:
NASAL CONGESTION
FEVER
ACHES & PAINS
SORE THROAT
COUGH12 FL OZ (354 mL) Orange Flavor
XL-3* Day Time
COLD MEDICINE
DO NOT USE IF PRINTED SHRINK BAND IS MISSING OR BROKEN
Failure to follow these warnings could result in serious consequencesDrug Facts
Active ingredients Purpose
(in each 15 mL, 1 tablespoon)
Acetaminophen 325 mg ........................ Pain reliever/fever reducer
Dextromethorphan HBr 10 mg .........................Cough Suppressant
Phenylephrine HCI 5 mg .................................Nasal decongestantUses temporarily relieves common cold and flu symptoms:
• nasal congestion • cough due to minor throat and bronchial
irritation • sore throat • headache • minor aches and pains • fever→ DRUG FACTS CONTINUED ON BACK →
Distributed by:
Midway Importing, Inc.
1807 Brittmoore Road,
Houston, TX 77043 1-800-531-3873
Made in USA
Peel
LR-059 REV 03 back ►XL-3* Day Time
COLD MEDICINE
Drug Facts (continued)
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if • adult takes more than 4,000 mg of acetaminophen in 24 hours • child takes more than 5 doses in 24 hours • taken with other drugs containing acetaminophen • adult has 3 or more alcoholic drinks every day while using this product.
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms
may include: • skin reddening • blisters • rash. If a skin reaction occurs,
stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, lasts for more than 2 days, is
accompanied or followed by fever, headache, rash, nausea, or vomiting,
consult a doctor promptly.Do not use • with any other products containing acetaminophen (prescription or nonprescription). lf you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist • for more than10 days for pain unless directed by a doctor • for more than 3 days for fever unless directed by a doctor • if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drugs. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have • liver disease
• heart disease • thyroid disease • diabetes • high blood pressure
• trouble urinating due to enlarged prostate gland • cough accompanied by
excessive phlegm (mucus) • persistent or chronic cough such as occurs with smoking, asthma, or emphysema.Ask a doctor or pharmacist before use if you are taking the blood thinning drug warfarin. ►
Drug Facts (continued)
When using this product • do not use more than directed (see
overdose warning) • avoid alcoholic drinksStop use and ask a doctor if • you get nervous, dizzy or sleepless
• symptoms get worse or last more than 5 days (children) or 7days (adults)
• fever gets worse or lasts more than 3 days • redness or swelling is
present • new symptoms occur • cough comes back, or occurs with
rash or headache that lasts. These could be signs of a serious condition.If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children.Overdose warning: Taking more than the recommended dose
(overdose) may cause serious health problems including liver damage.
In case of accidental overdose, get medical help or contact a Poison
Control Center (1-800-222-1222) right away.
Prompt medical attention is critical for adults as well as children even if
you do not notice any signs or symptoms.Directions • take only as directed - see Overdose warning
• use dose cup or tablespoon • do not exceed 4 doses per 24 hours
• if taking Day Time during the day and Night Time at night, limit total to
4 doses per 24 hours
___________________________________________________________
adults & children 12 years and over │ 30 mL (2 TBSP) every 4 hours
___________________________________________________________
children 6 to under 12 years │ 15mL (1TBSP) every 4 hours
___________________________________________________________
children 4 to under 6 years │ ask a doctor
___________________________________________________________
children under 4 years │ do not use
___________________________________________________________
Other information • Sodium content per tablespoon: 10mg
• store at 20°-25°C (68°-77° F).Inactive ingredients citric acid, FD & C Yellow No.6, flavor, glycerin,
propylene glycol, purified water, saccharin sodium, sodium benzoate, sucrose.Questions or Comments? Call weekdays from 9:30 AM to 4:30 PM EST
1-877-798-5944
XL-3*
Day Time
Non-Drowsy Cold/Flu reliefCOLD MEDICINE
• Acetaminophen - Pain Reliever/Fever Reducer
• Dextromethorphan HBr - Cough Suppressant
• Phenylephrine HCI - Nasal DecongestantFast Relief of:
NASAL CONGESTION
FEVER
ACHES & PAINS
SORE THROAT
COUGH6 FL OZ (177 mL) Orange Flavor

-
INGREDIENTS AND APPEARANCE
XL-3 DAY TIME
acetaminophen, dextromethorphan hbr, phenylephrine hcl liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76281-304 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg in 15 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 15 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE 5 mg in 15 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BEHENATE (UNII: H4W8BMK72F) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76281-304-28 354 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/01/2018 XL-3 DAY TIME
acetaminophen, dextromethorphan hbr, phenylephrine hcl liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76281-306 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg in 15 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg in 15 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE 5 mg in 15 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GLYCERIN (UNII: PDC6A3C0OX) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BEHENATE (UNII: H4W8BMK72F) SUCROSE (UNII: C151H8M554) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76281-306-25 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/01/2018 Labeler - AptaPharma Inc. (790523323) Registrant - AptaPharma Inc. (790523323) Establishment Name Address ID/FEI Business Operations AptaPharma Inc. 790523323 manufacture(76281-304, 76281-306)



