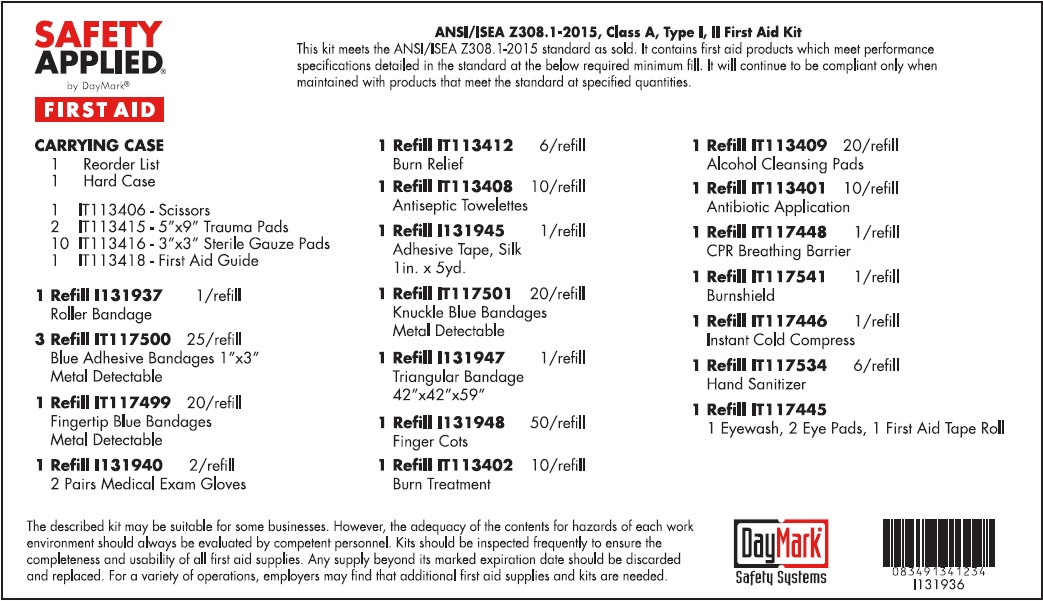
Label: STANDARD ANSI FIRST AID- water, benzalkonium chloride, isopropyl alcohol, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, lidocaine hydrochloride kit
-
NDC Code(s):
50814-010-01,
50814-011-01,
50814-012-01,
50814-013-01, view more50814-014-01, 50814-015-01, 50814-020-01
- Packager: GFA Production (Xiamen) Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated March 6, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Use
-
Warnings
For external use only.
When using this product
• to avoid contamination, do not touch tip of container to any surface • do not reuse • once opened, discard • obtain immediate medical treatment for all open wounds in or near the eyes
- Directions
- Other information
- Inactive ingredients
- DRUG FACTS
- Active Ingredient:
- Use:
- Warnings:
- Directions:
- Inactive ingredient:
- DRUG FACTS
- Active Ingredient:
- Use:
- Warnings:
- Directions:
- Other information:
- Inactive ingredient:
- Drug Facts
- Active ingredients (in each gram)
- Use
- Warnings
- Directions
- Other information
- Inactive ingredients
- Drug Facts
- Active ingredients
- Uses
-
Warnings
For external use only.
Do not use
• in the eyes • over large areas of the body • in large quantities • over raw surfaces or blistered areas • longer than 1 week unless directed by a doctor
- Directions
- Other information
- Inactive ingredients
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Eye Wash (50814-010-01) Labeling:
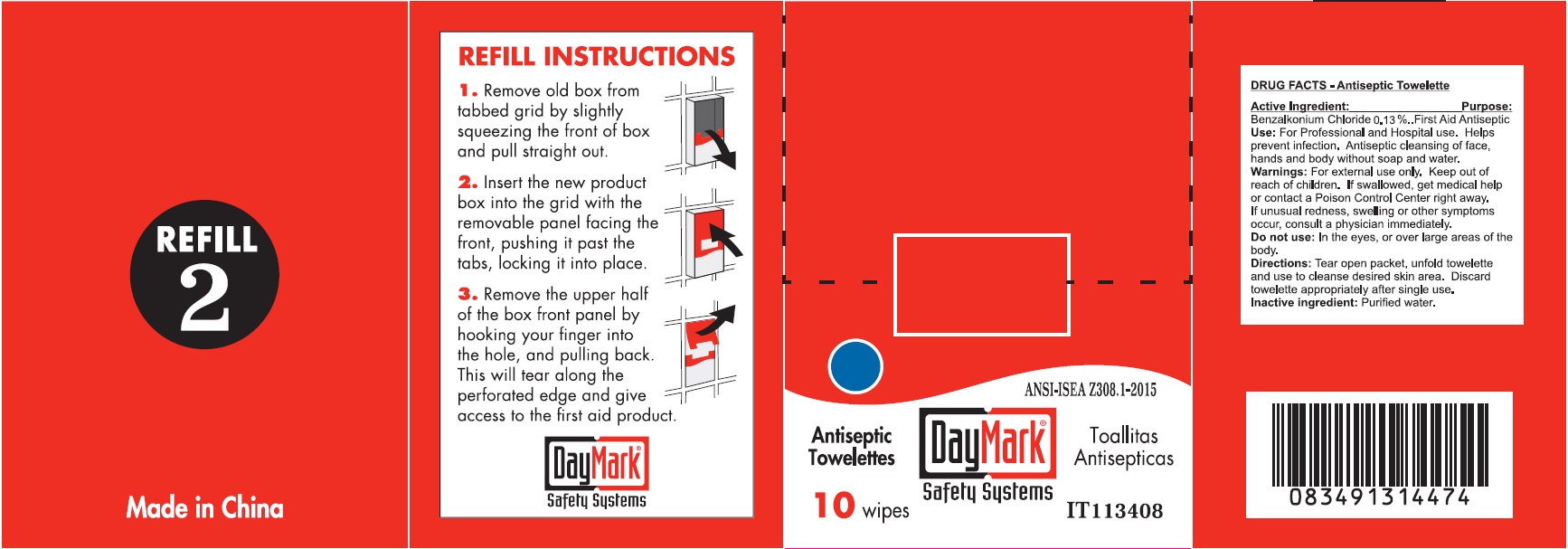
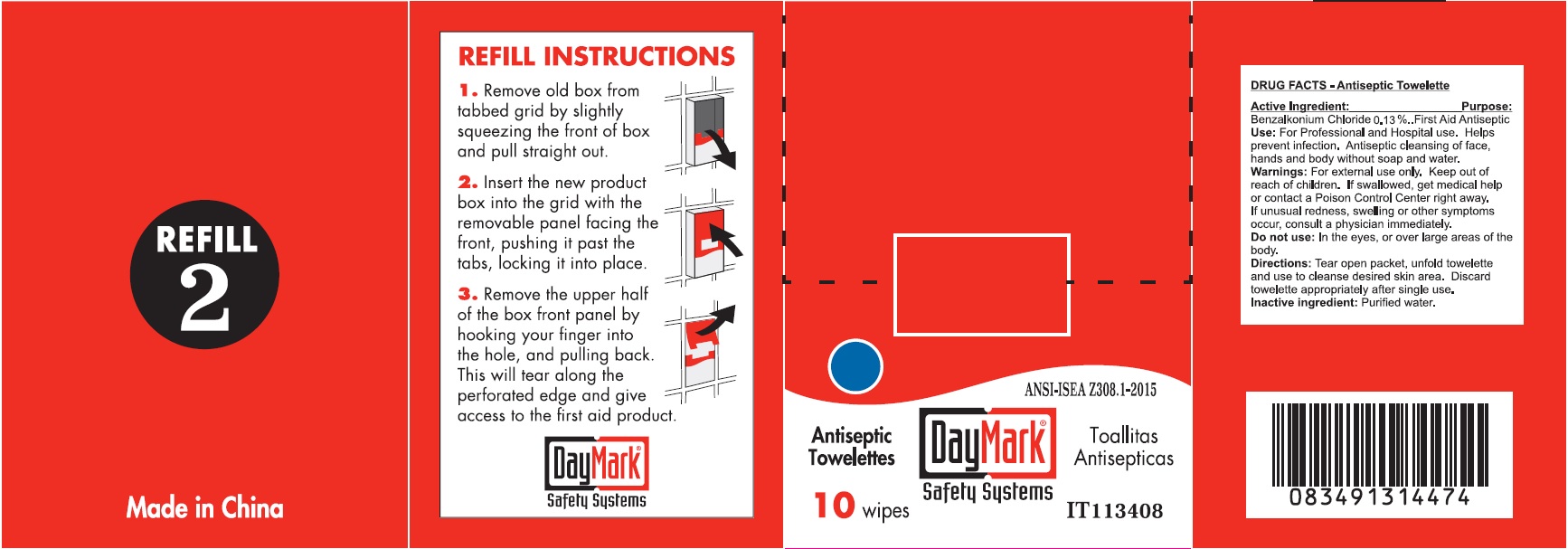
- Antiseptic Towelettes (50814-011-01) Labeling:
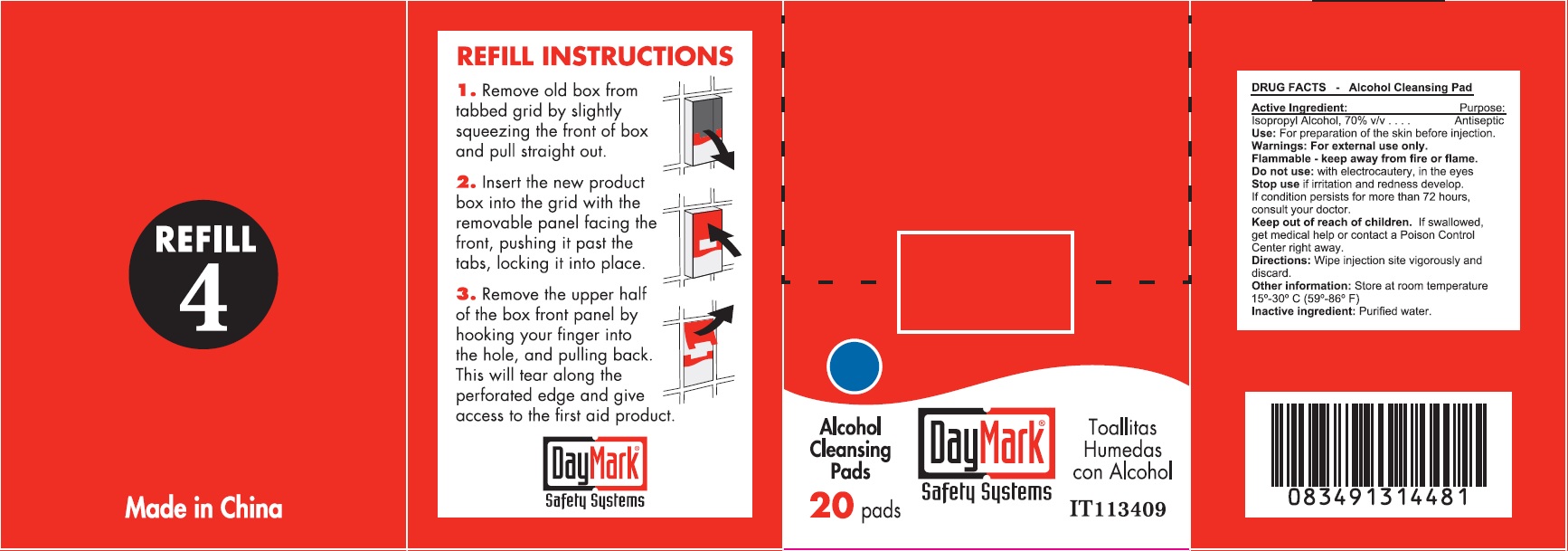
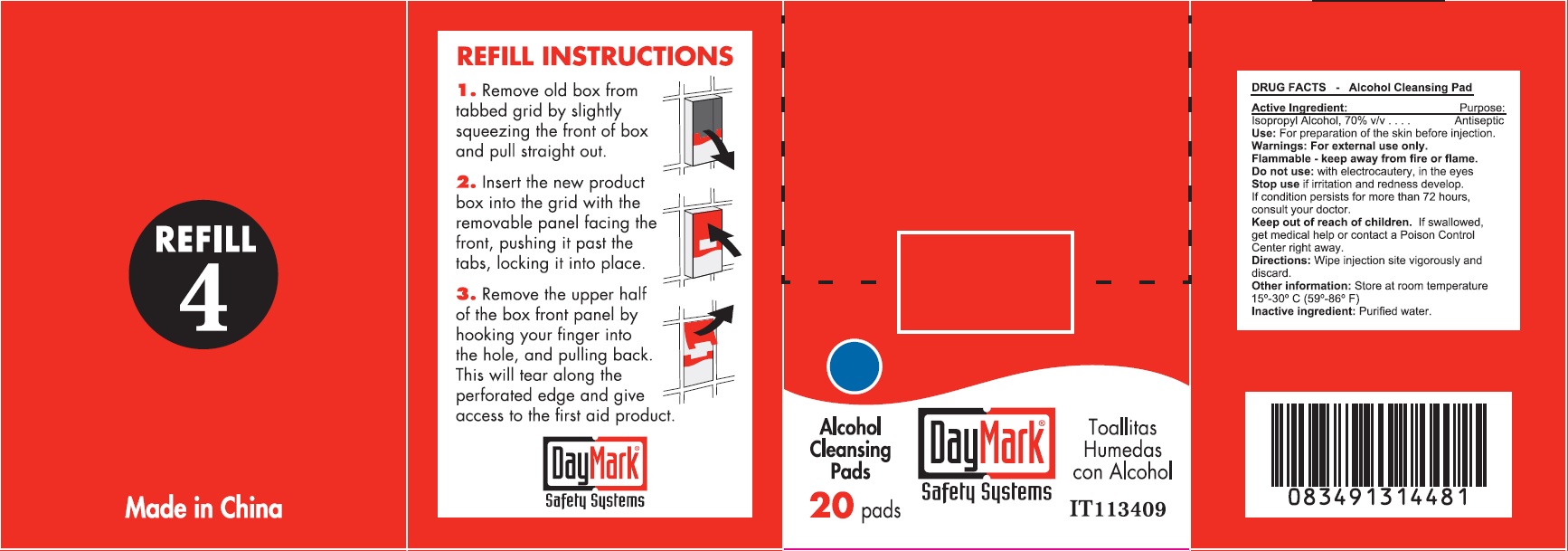
- Alcohol Cleansing Pads (50814-012-01) Labeling:
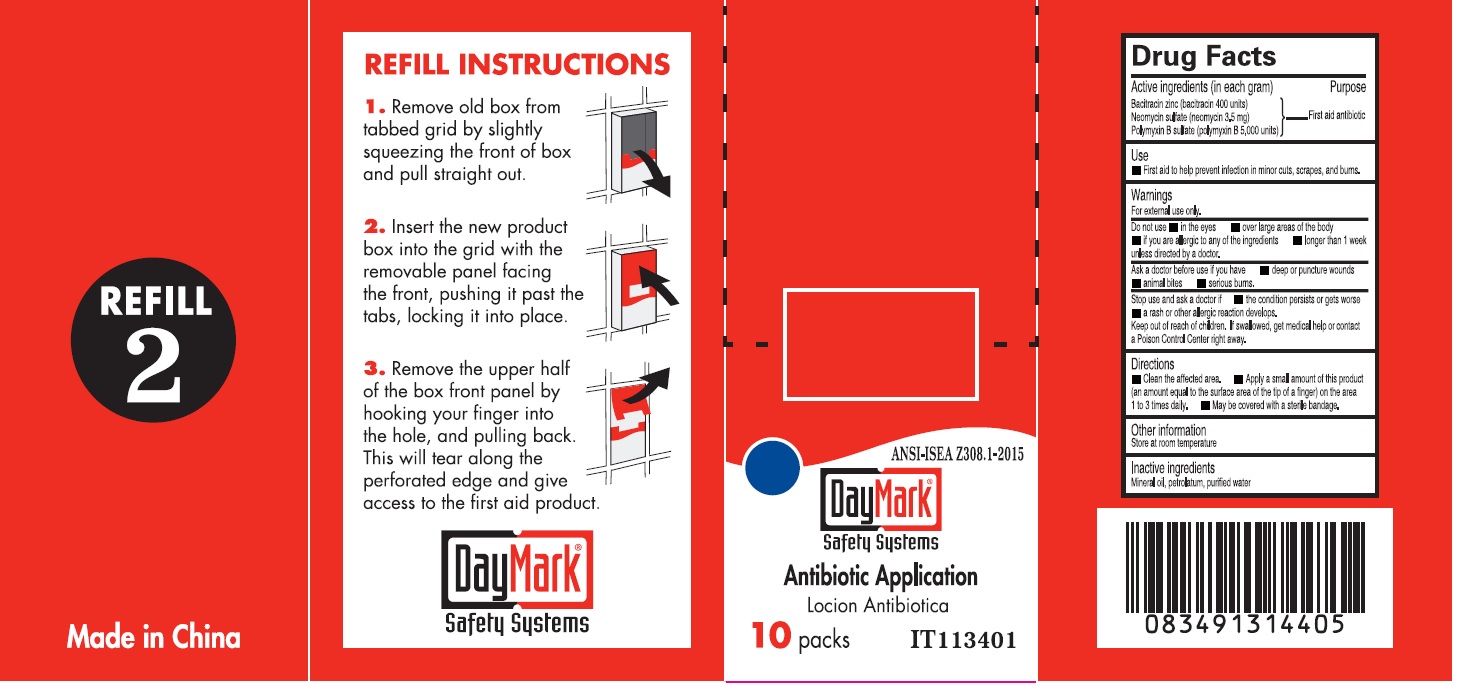
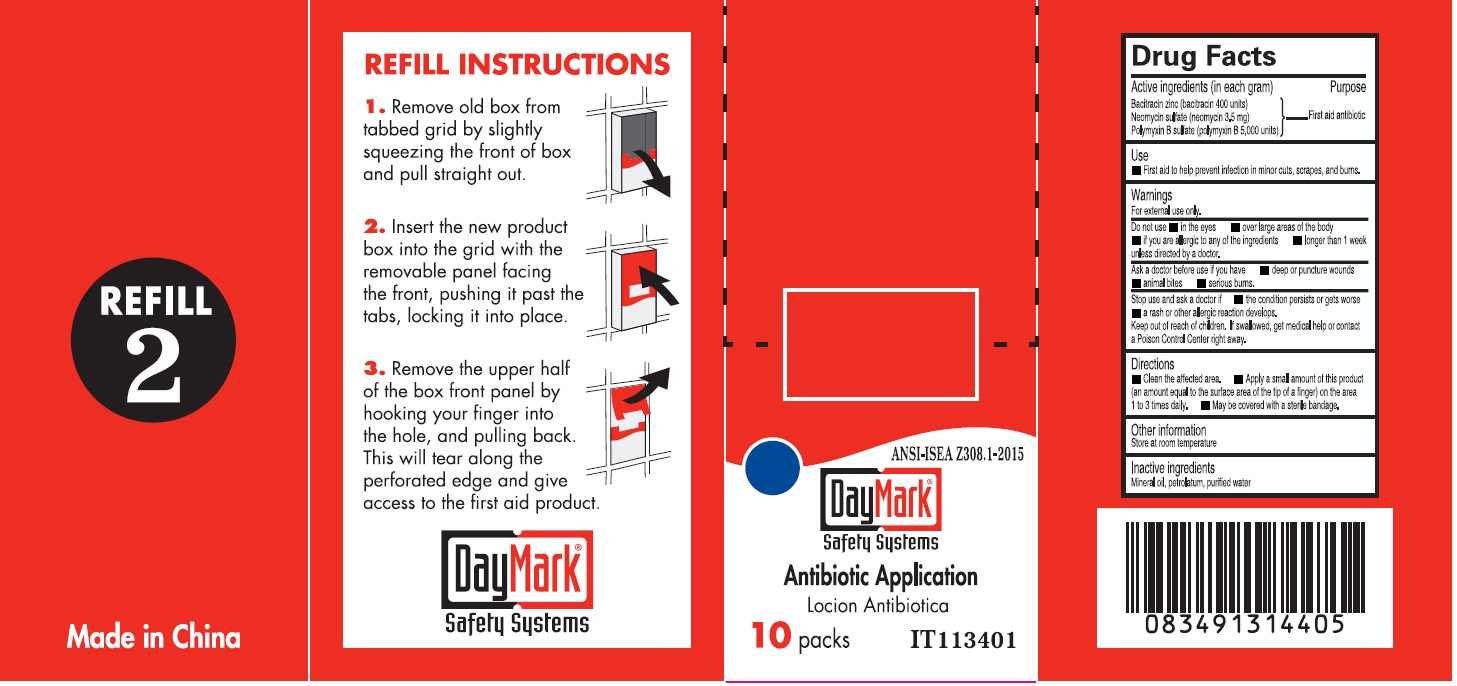
- Antibiotic Application (50814-013-01) Labeling:
- Burn Treatment (50814-014-01) Labeling:
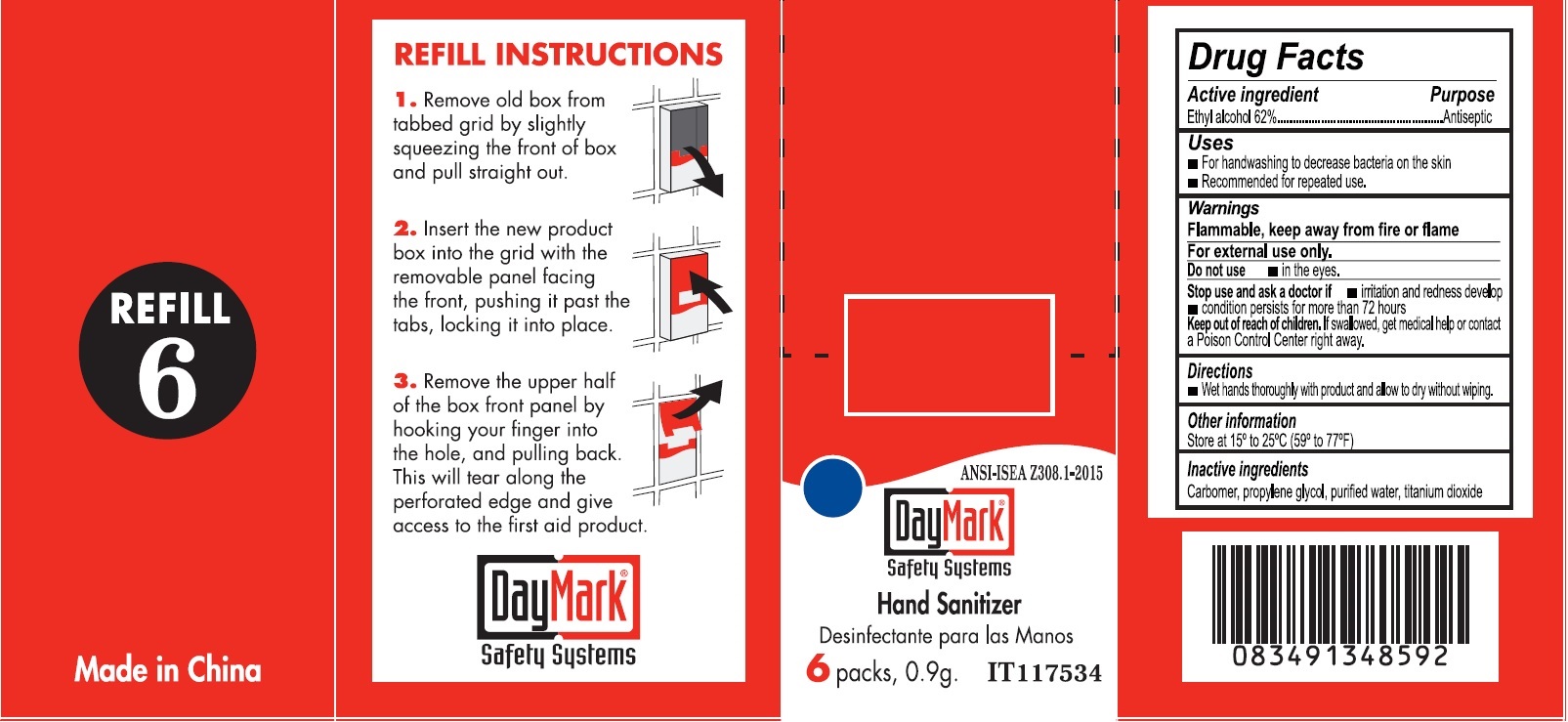
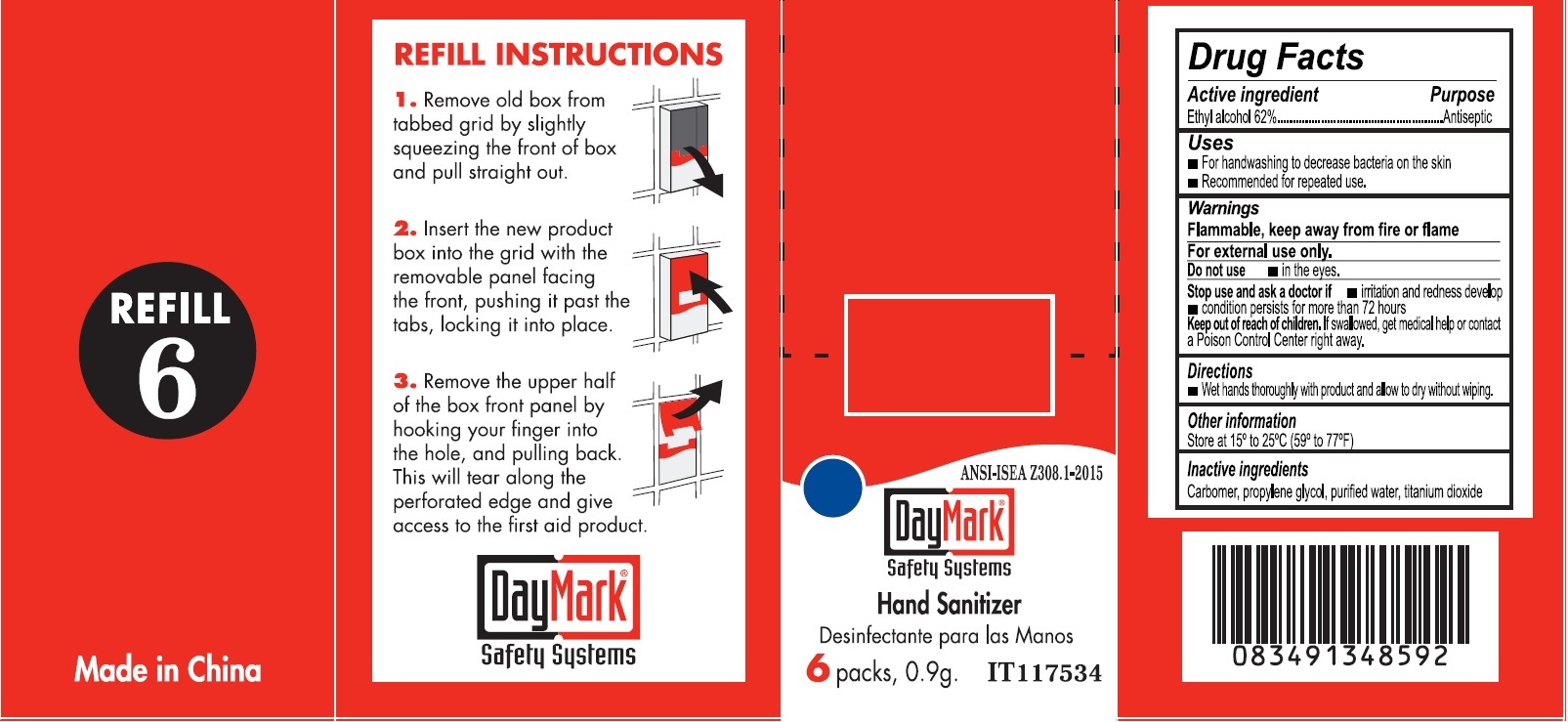
- Hand Sanitizer (50814-015-01) Labeling:
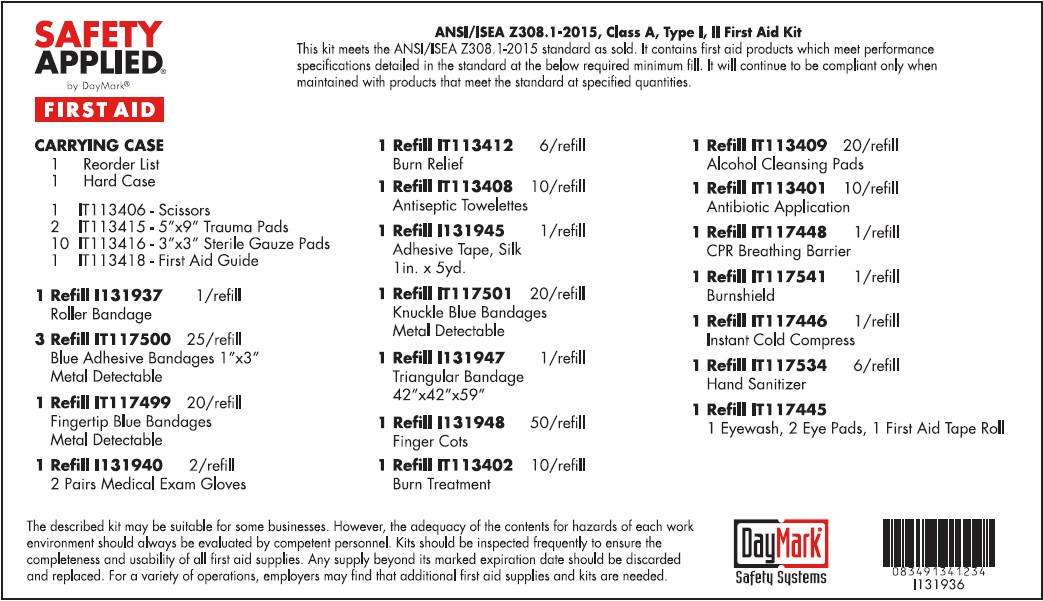
- Standard ANSI First Aid (50814-020-01) Labeling:
-
INGREDIENTS AND APPEARANCE
STANDARD ANSI FIRST AID
water, benzalkonium chloride, isopropyl alcohol, bacitracin zinc, neomycin sulfate, polymyxin b sulfate, lidocaine hydrochloride kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50814-020 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50814-020-01 1 in 1 KIT 08/10/2016 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 30 mL Part 2 10 PATCH 9 g Part 3 10 PATCH 9 g Part 4 6 PACKAGE 5 g Part 5 10 PACKAGE 9 g Part 6 6 PACKAGE 5.4 g Part 1 of 6 EYE WASH
water solutionProduct Information Item Code (Source) NDC:50814-010 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength WATER (UNII: 059QF0KO0R) (WATER - UNII:059QF0KO0R) WATER 991 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) SODIUM CHLORIDE (UNII: 451W47IQ8X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50814-010-01 1 in 1 BOX 1 30 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part349 08/10/2016 Part 2 of 6 ANTISEPTIC TOWELETTES
benzalkonium chloride clothProduct Information Item Code (Source) NDC:50814-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50814-011-01 1 in 1 BOX 1 10 in 1 BOX 1 0.9 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/10/2016 Part 3 of 6 ALCOHOL CLEANSING
isopropyl alcohol clothProduct Information Item Code (Source) NDC:50814-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ISOPROPYL ALCOHOL (UNII: ND2M416302) (ISOPROPYL ALCOHOL - UNII:ND2M416302) ISOPROPYL ALCOHOL 70 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50814-012-01 1 in 1 BOX 1 10 in 1 BOX 1 0.9 g in 1 PATCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/10/2016 Part 4 of 6 ANTIBIOTIC APPLICATION
bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointmentProduct Information Item Code (Source) NDC:50814-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [iU] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 3.5 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [iU] in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50814-013-01 1 in 1 BOX 1 10 in 1 BOX 1 0.9 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 08/10/2016 Part 5 of 6 BURN TREATMENT
benzalkonium chloride, lidocaine hydrochloride creamProduct Information Item Code (Source) NDC:50814-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 0.5 g in 100 g Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50814-014-01 1 in 1 BOX 1 10 in 1 BOX 1 0.9 g in 1 PACKAGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/10/2016 Part 6 of 6 HAND SANITIZER
alcohol gelProduct Information Item Code (Source) NDC:50814-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.62 g in 1 g Inactive Ingredients Ingredient Name Strength CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50814-015-01 1 in 1 BOX 1 6 in 1 BOX 1 0.9 g in 1 PACKAGE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 08/10/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 08/10/2016 Labeler - GFA Production (Xiamen) Co., Ltd. (421256261) Establishment Name Address ID/FEI Business Operations GFA Production (Xiamen) Co., Ltd. 421256261 manufacture(50814-020, 50814-010, 50814-011, 50814-012, 50814-013, 50814-014, 50814-015)