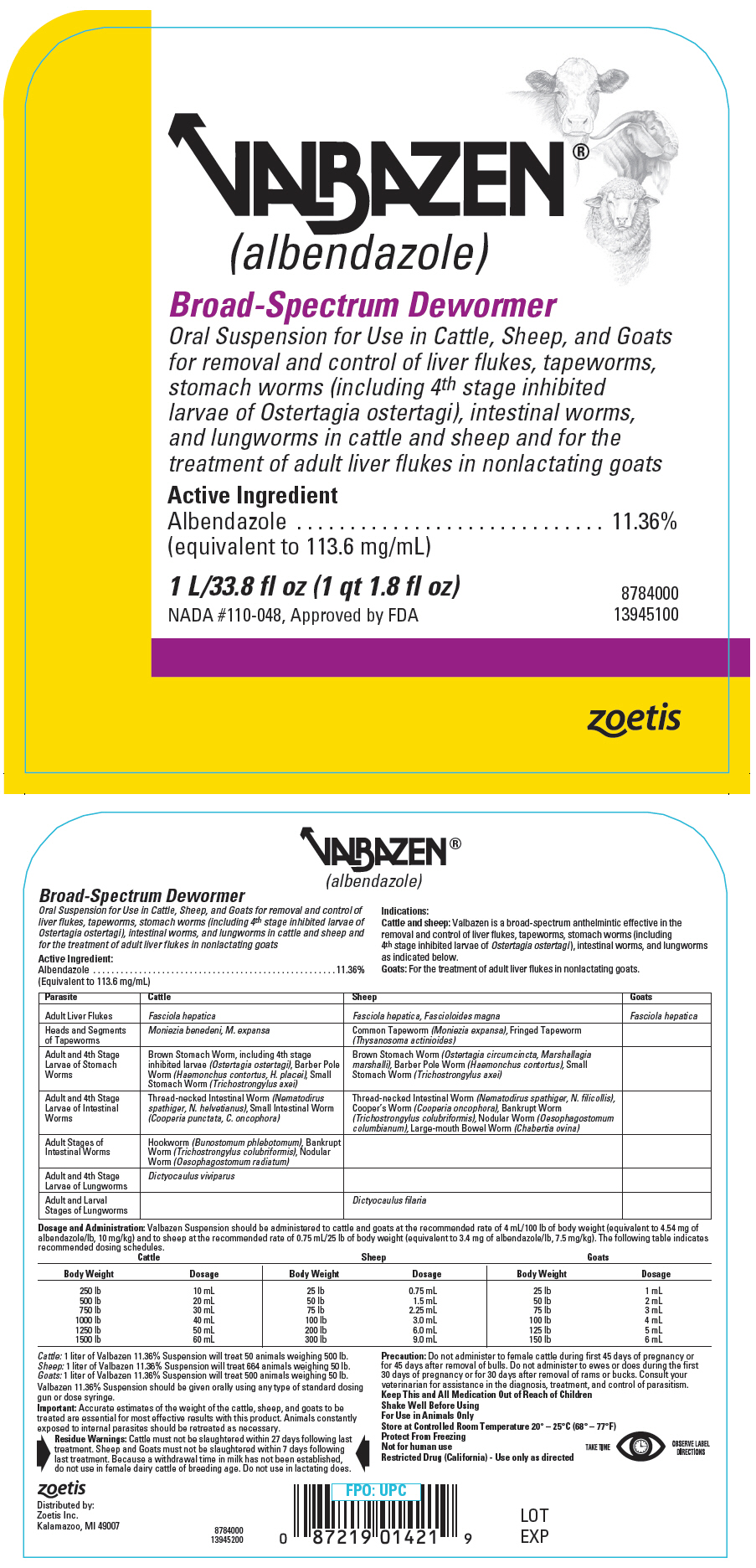
Label: VALBAZEN- albendazole suspension
- NDC Code(s): 54771-8783-1, 54771-8783-2, 54771-8783-3
- Packager: Zoetis Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated June 26, 2020
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
VALBAZEN®
(albendazole oral suspension)Broad-Spectrum Dewormer
Oral Suspension for Use in Cattle, Sheep, and Goats for removal and control of liver flukes, tapeworms, stomach worms (including 4th stage inhibited larvae of Ostertagia ostertagi), intestinal worms, and lungworms in cattle and sheep and for the treatment of adult liver flukes in nonlactating goats. - SPL UNCLASSIFIED SECTION
-
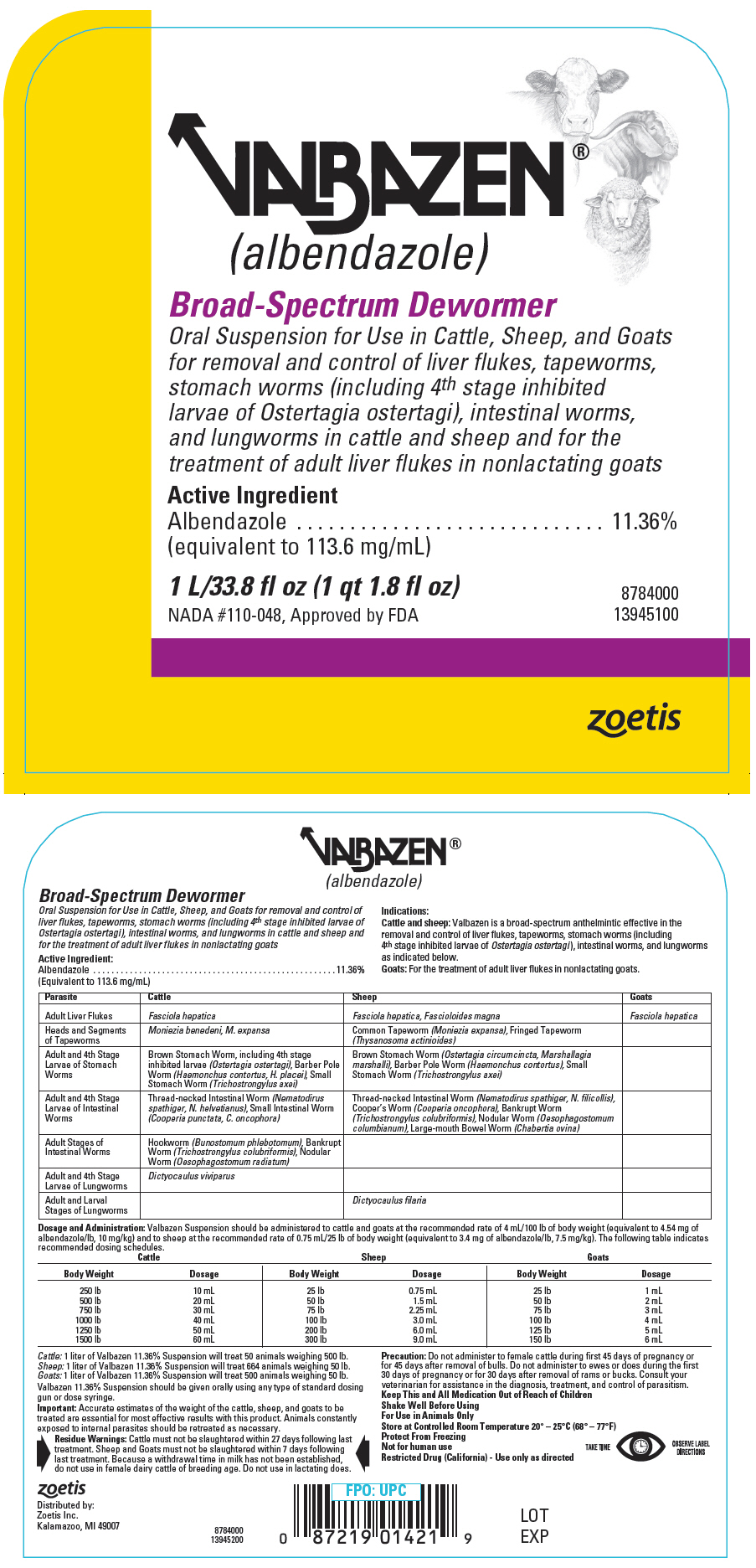
Indications
Cattle and sheep: Valbazen is a broad-spectrum anthelmintic effective in the removal and control of liver flukes, tapeworms, stomach worms (including 4th stage inhibited larvae of Ostertagia ostertagi), intestinal worms, and lungworms as indicated below.
Goats: For the treatment of adult liver flukes in nonlactating goats.
Parasite Cattle Sheep Goats Adult Liver Flukes Fasciola hepatica Fasciola hepatica, Fascioloides magna Fasciola hepatica Heads and Segments of Tapeworms Moniezia benedeni, M. expansa Common Tapeworm (Moniezia expansa), Fringed Tapeworm (Thysanosoma actinioides) Adult and 4th Stage Larvae of Stomach Worms Brown Stomach Worm, including 4th stage inhibited larvae (Ostertagia ostertagi), Barber Pole Worm (Haemonchus contortus, H. placei), Small Stomach Worm (Trichostrongylus axei) Brown Stomach Worm (Ostertagia circumcincta, Marshallagia marshalli), Barber Pole Worm (Haemonchus contortus), Small Stomach Worm (Trichostrongylus axei) Adult and 4th Stage Larvae of Intestinal Worms Thread-necked Intestinal Worm (Nematodirus spathiger, N. helvetianus), Small Intestinal Worm (Cooperia punctata, C. oncophora) Thread-necked Intestinal Worm (Nematodirus spathiger, N. filicollis), Cooper's Worm (Cooperia oncophora), Bankrupt Worm (Trichostrongylus colubriformis), Nodular Worm (Oesophagostomum columbianum), Large-mouth Bowel Worm (Chabertia ovina) Adult Stages of Intestinal Worms Hookworm (Bunostomum phlebotomum), Bankrupt Worm (Trichostrongylus colubriformis), Nodular Worm (Oesophagostomum radiatum) Adult and 4th Stage Larvae of Lungworms Dictyocaulus viviparus Adult and Larval Stages of Lungworms Dictyocaulus filaria -
Dosage and Administration
Valbazen Suspension should be administered to cattle and goats at the recommended rate of 4 mL/100 lb of body weight (equivalent to 4.54 mg of albendazole/lb, 10 mg/kg) and to sheep at the recommended rate of 0.75 mL/25 lb of body weight (equivalent to 3.4 mg of albendazole/lb, 7.5 mg/kg). The following table indicates recommended dosing schedules. Do not underdose. Ensure each animal receives a complete dose based on a current body weight. Underdosing may result in ineffective treatment, and encourage the development of parasite resistance.
Cattle Sheep Goats Body Weight Dosage Body Weight Dosage Body Weight Dosage 250 lb 10 mL 25 lb 0.75 mL 25 lb 1 mL 500 lb 20 mL 50 lb 1.5 mL 50 lb 2 mL 750 lb 30 mL 75 lb 2.25 mL 75 lb 3 mL 1000 lb 40 mL 100 lb 3.0 mL 100 lb 4 mL 1250 lb 50 mL 200 lb 6.0 mL 125 lb 5 mL 1500 lb 60 mL 300 lb 9.0 mL 150 lb 6 mL Cattle: 1 liter of Valbazen 11.36% Suspension will treat 50 animals weighing 500 lb.
Sheep: 1 liter of Valbazen 11.36% Suspension will treat 664 animals weighing 50 lb.
Goats: 1 liter of Valbazen 11.36% Suspension will treat 500 animals weighing 50 lb.
Valbazen 11.36% Suspension should be given orally using any type of standard dosing gun or dose syringe.
- Important
- Residue Warnings
-
Other Warnings
Parasite resistance may develop to any dewormer, and has been reported for most classes of dewormers. Treatment with a dewormer used in conjunction with parasite management practices appropriate to the geographic area and the animal(s) to be treated may slow the development of parasite resistance. Fecal examinations or other diagnostic tests and parasite management history should be used to determine if the product is appropriate for the herd/flock, prior to the use of any dewormer. Following the use of any dewormer, effectiveness of treatment should be monitored (for example, with the use of a fecal egg count reduction test or another appropriate method). A decrease in a drug’s effectiveness over time as calculated by fecal egg count reduction tests may indicate the development of resistance to the dewormer administered. Your parasite management plan should be adjusted accordingly based on regular monitoring.
-
Precaution
Do not administer to female cattle during first 45 days of pregnancy or for 45 days after removal of bulls. Do not administer to ewes or does during the first 30 days of pregnancy or for 30 days after removal of rams or bucks. Consult your veterinarian for assistance in the diagnosis, treatment, and control of parasitism.
- SPL UNCLASSIFIED SECTION
- STORAGE AND HANDLING
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 1 L/33.8 fl oz Bottle Label
VALBAZEN®
(albendazole)Broad-Spectrum Dewormer
Oral Suspension for Use in Cattle, Sheep, and Goats
for removal and control of liver flukes, tapeworms,
stomach worms (including 4th stage inhibited
larvae of Ostertagia ostertagi), intestinal worms,
and lungworms in cattle and sheep and for the
treatment of adult liver flukes in nonlactating goatsActive Ingredient
Albendazole
(equivalent to 113.6 mg/mL)
11.36%1 L/33.8 fl oz (1 qt 1.8 fl oz)
NADA #110-048, Approved by FDA
8784000
13945100zoetis
-
PRINCIPAL DISPLAY PANEL - 5 L/169 fl oz Bottle Label
VALBAZEN®
(albendazole)Broad-Spectrum Dewormer
Oral Suspension for Use in Cattle, Sheep, and Goats
for removal and control of liver flukes, tapeworms,
stomach worms (including 4th stage inhibited
larvae of Ostertagia ostertagi), intestinal worms,
and lungworms in cattle and sheep and for the
treatment of adult liver flukes in nonlactating goatsActive Ingredient
Albendazole
(equivalent to 113.6 mg/mL)
11.36%5 L/169 fl oz (1 gal 1 qt 9 fl oz)
NADA #110-048, Approved by FDA
8785000
13945600zoetis

-
PRINCIPAL DISPLAY PANEL - 500 mL/16.9 fl oz Bottle Label
VALBAZEN®
(albendazole)Broad-Spectrum Dewormer
Oral Suspension for Use in Cattle, Sheep, and Goats
for removal and control of liver flukes, tapeworms,
stomach worms (including 4th stage inhibited larvae of
Ostertagia ostertagi), intestinal worms, and lungworms
in cattle and sheep and for the treatment of adult liver
flukes in nonlactating goatsActive Ingredient:
Albendazole
(Equivalent to 113.6 mg/mL)
11.36%500 mL/16.9 fl oz (1 pt 1 fl oz)
NADA #110-048, Approved by FDA
zoetis

-
INGREDIENTS AND APPEARANCE
VALBAZEN
albendazole suspensionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:54771-8783 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength albendazole (UNII: F4216019LN) (albendazole - UNII:F4216019LN) albendazole 113.6 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) 6 mg in 1 mL GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYSORBATE 80 (UNII: 6OZP39ZG8H) BENZOIC ACID (UNII: 8SKN0B0MIM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54771-8783-1 500 mL in 1 BOTTLE 2 NDC:54771-8783-2 1000 mL in 1 BOTTLE 3 NDC:54771-8783-3 5000 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA110048 06/13/1989 Labeler - Zoetis Inc. (828851555)