Label: ERY-PED- erythromycin ethylsuccinate suspension
- NDC Code(s): 80005-155-18
- Packager: Carnegie Pharmaceuticals LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated December 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTIONRx only - To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ery-Ped and other antibacterial drugs, Ery-Ped should be used only to treat or prevent infections ...
-
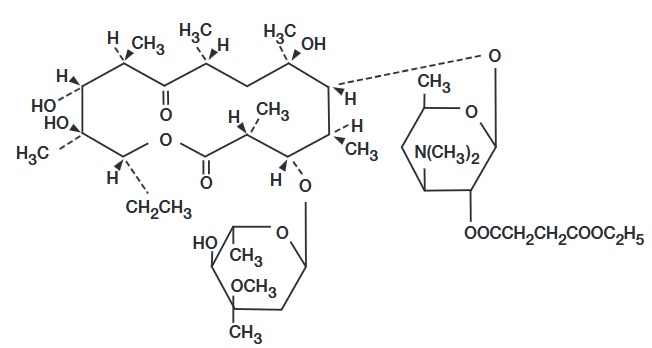
DESCRIPTIONErythromycin is produced by a strain of Saccharopolyspora erythraea (formerly Streptomyces erythraeus) and belongs to the macrolide group of antibiotics. It is basic and readily forms salts with ...
-
CLINICAL PHARMACOLOGYOrally administered erythromycin ethylsuccinate suspension is readily and reliably absorbed under both fasting and nonfasting conditions. Erythromycin diffuses readily into most body fluids. Only ...
-
INDICATIONS AND USAGETo reduce the development of drug-resistant bacteria and maintain the effectiveness of Ery-Ped and other antibacterial drugs, Ery-Ped should be used only to treat or prevent infections that are ...
-
CONTRAINDICATIONSErythromycin is contraindicated in patients with known hypersensitivity to this antibiotic. Erythromycin is contraindicated in patients taking terfenadine, astemizole, pimozide, or cisapride (see ...
-
WARNINGSHepatotoxicity: There have been reports of hepatic dysfunction, including increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice, occurring in ...
-
PRECAUTIONSGeneral: Prescribing Ery-Ped in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the ...
-
ADVERSE REACTIONSThe most frequent side effects of oral erythromycin preparations are gastrointestinal and are dose-related. They include nausea, vomiting, abdominal pain, diarrhea and anorexia. Symptoms of ...
-
OVERDOSAGEIn case of overdosage, erythromycin should be discontinued. Overdosage should be handled with the prompt elimination of unabsorbed drug and all other appropriate measures should be ...
-
DOSAGE AND ADMINISTRATIONEry-Ped (erythromycin ethylsuccinate) oral suspensions may be administered without regard to meals. Children: Age, weight, and severity of the infection are important factors in determining ...
-
HOW SUPPLIEDEry-Ped 200 (erythromycin ethylsuccinate for oral suspension, USP) is supplied in bottles of 100 mL (NDC 80005-154-18). Ery-Ped 400 (erythromycin ethylsuccinate for oral suspension, USP) is ...
-
REFERENCESCommittee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, the American Heart Association: Prevention of Rheumatic Fever. Circulation ...
-
SPL UNCLASSIFIED SECTIONRev. 12/2024 - Carnegie Pharmaceuticals LLC - Delran, NJ 08075, USA
-
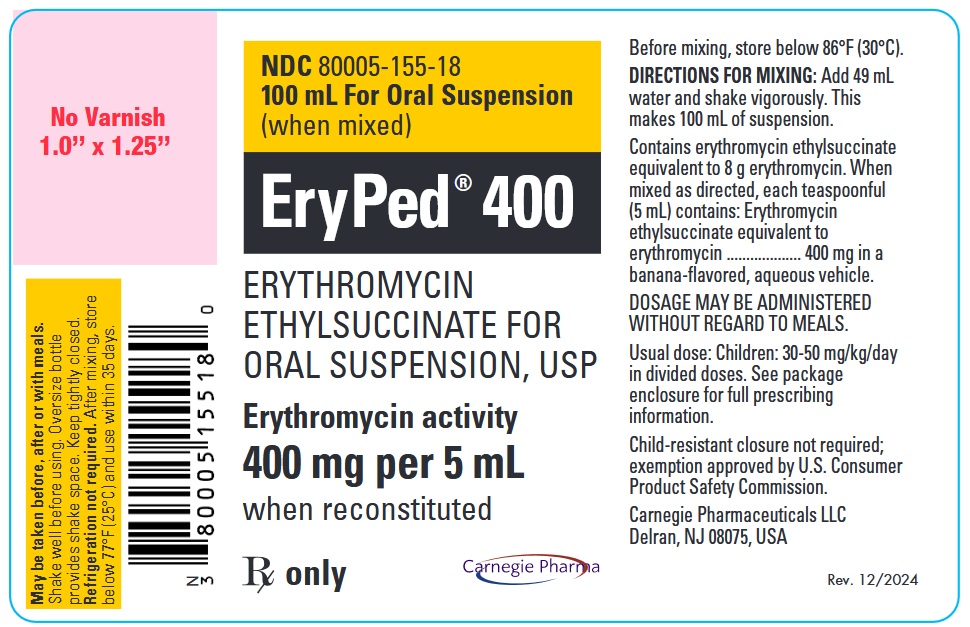
PRINCIPAL DISPLAY PANEL - 400 mg per 5 mL Bottle LabelNDC 80005-155-18 - 100 mL For Oral Suspension - (when mixed) EryPed® 400 - ERYTHROMYCIN - ETHYLSUCCINATE FOR - ORAL SUSPENSION, USP - Erythromycin activity - 400 mg per 5 mL - when ...
-
INGREDIENTS AND APPEARANCEProduct Information