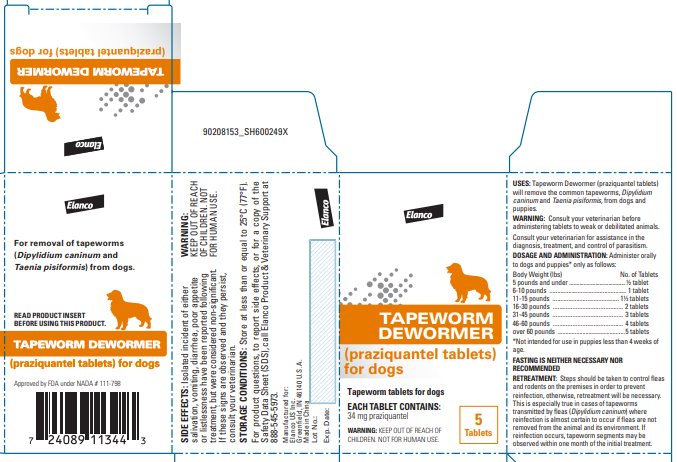
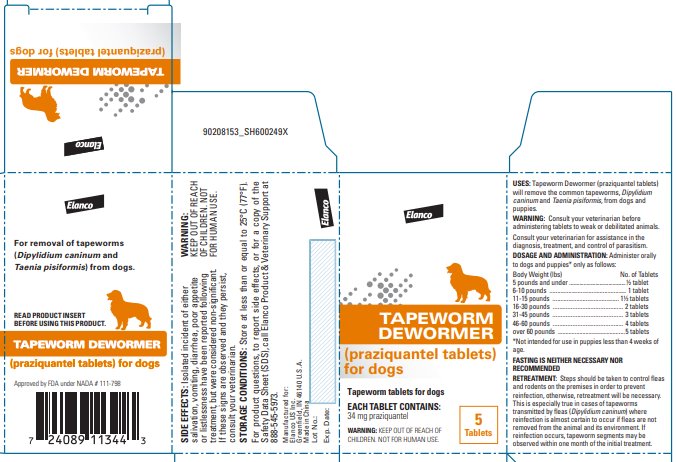
Label: TAPEWORM DEWORMER FOR DOGS- praziquantel tablet
- NDC Code(s): 58198-0064-1, 58198-0064-2
- Packager: Elanco US Inc.
- Category: OTC ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Animal Drug Application
Drug Label Information
Updated October 24, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- DESCRIPTION:
- USES:
-
DOSAGE AND ADMINISTRATION:
Tapeworm Dewormer (praziquantel tablets) may be given directly in the mouth or crumbled and mixed with the food. Administer to dogs and puppies* only as follows:
*Not intended for use in puppies less than 4 weeks of age. Body Weight (lbs)
No. of Tablets
5 lbs. and under
½ tablet
6-10 lbs.
1 tablet
11-15 lbs.
1½ tablets
16-30 lbs.
2 tablets
31-45 lbs.
3 tablets
46-60 lbs.
4 tablets
Over 60 lbs.
5 tablets maximum
FASTING IS NEITHER NECESSARY NOR RECOMMENDED.
-
RETREATMENT:
Steps should be taken to control fleas and rodents on the premises in order to prevent reinfection; otherwise, retreatment will be necessary. This is especially true in cases of tapeworms transmitted by fleas (Dipylidium caninum) where reinfection is almost certain to occur if fleas are not removed from the animal and its environment. If reinfection occurs, tapeworm segments may be observed within one month of the initial treatment.
- SIDE EFFECTS:
-
Questions?
For product questions, to report side effects, or for a copy of the Safety Data Sheet (SDS), call Elanco Product & Veterinary Support at 888-545-5973.
For additional information about reporting side effects for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
- WARNING:
- HOW SUPPLIED:
-
STORAGE CONDITIONS:
Store at less than or equal to 25°C (77°F).
Elanco™
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140 U.S.A.Approved by FDA under NADA # 111-798
Elanco and the diagonal bar logo are trademarks of Elanco or
its affiliates.Made in China Revised: March 2022
90208154_PA600249X
© 2022 Elanco or its affiliates.
- Principal Display Panel - 34 mg Carton Label
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TAPEWORM DEWORMER FOR DOGS
praziquantel tabletProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:58198-0064 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PRAZIQUANTEL (UNII: 6490C9U457) (PRAZIQUANTEL - UNII:6490C9U457) PRAZIQUANTEL 34 mg Product Characteristics Color white (white) Score 2 pieces Shape ROUND (ROUND) Size 13mm Flavor Imprint Code 34 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58198-0064-1 1 in 1 CARTON 1 NDC:58198-0064-2 5 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA111798 05/16/2014 Labeler - Elanco US Inc. (966985624) Establishment Name Address ID/FEI Business Operations TriRx Shawnee LLC 118187894 MANUFACTURE, ANALYSIS, PACK, LABEL Establishment Name Address ID/FEI Business Operations Merck KG auf Aktien 342249299 API MANUFACTURE Establishment Name Address ID/FEI Business Operations Hisun Pharmaceutical (Nantong) Co., Ltd. 421314476 API MANUFACTURE