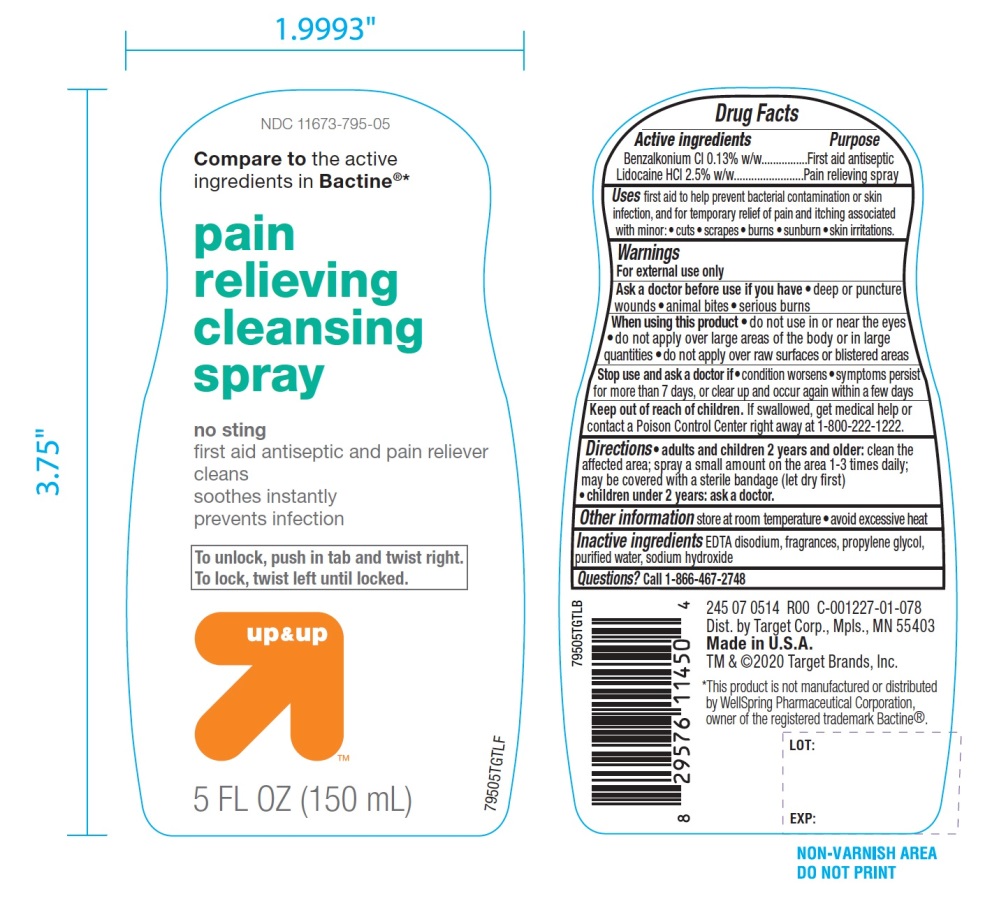
Label: PAIN RELIEVING CLEANSING- benzalkonium chloride, lidocaine hcl spray
- NDC Code(s): 11673-795-05
- Packager: TARGET CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 16, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Uses
-
Warnings
For external use only
When using this product
- •
- do not use in or near the eyes
- •
- do not apply over large area of the body or in large quantities
- •
- do not apply over raw surfaces or blistered areas
- Directions
- Other information
- Inactive ingredients
-
Principal Display Panel/Package Label
NDC# 11673-795-05
Compare to the active ingredients in Bactine®*
Pain Relieving
Cleansing Spray
NO STING
First Aid Antiseptic & Pain Reliever
- •
- Cleans
- •
- Soothes Instantly
- •
- Prevents Infection
To unlock, push in tab and twist right. To lock, twist left until locked.
up & up
5 FL OZ (150 mL)
DIST. by Target Corp.
Mpls., MN 55403
Made in U.S.A.
TM & ©2020 Target Brands, Inc.
*This product is not manufactured or distributed by Well Spring Pharmaceuticals Corporation, owner of the registered trademark Bactine®.
-
INGREDIENTS AND APPEARANCE
PAIN RELIEVING CLEANSING
benzalkonium chloride, lidocaine hcl sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-795 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 mL LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE 2.5 g in 100 mL Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Propylene Glycol (UNII: 6DC9Q167V3) Water (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-795-05 150 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 03/10/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 03/10/2020 Labeler - TARGET CORPORATION (006961700)