Label: NITROFURANTION capsule
- NDC Code(s): 55700-893-14
- Packager: Quality Care Products, LLC
- This is a repackaged label.
- Source NDC Code(s): 57664-233
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 8, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nitrofurantoin Capsules, USP (macrocrystals) and other antibacterial drugs, Nitrofurantoin Capsules, USP (macrocrystals) should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
-
DESCRIPTION
Nitrofurantoin macrocrystals, USP is a synthetic chemical of controlled crystal size. It is a stable, yellow, crystalline compound. Nitrofurantoin macrocrystals, USP is an antibacterial agent for specific urinary tract infections. It is available in 25 mg, 50 mg, and 100 mg capsules for oral administration.
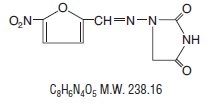
It is chemically designated as 1-[[(5-nitro-2-furanyl)methylene] amino]-2,4-imidazolidinedione and has the following structural formula:
Each Nitrofurantoin Capsules, USP (macrocrystals) contains nitrofurantoin (macrocrystals) USP equivalent to 25 mg, 50 mg or 100 mg and the following inactive ingredients: lactose monohydrate, corn starch, talc, titanium dioxide, gelatin, sodium lauryl sulfate, shellac, propylene glycol, potassium hydroxide and black iron oxide. In addition 50 mg capsules contain FD&C Blue #1, red iron oxide and yellow iron oxide.
-
CLINICAL PHARMACOLOGY:
Nitrofurantoin macrocrystals are a larger crystal form of nitrofurantoin. The absorption of Nitrofurantoin macrocrystals is slower and its excretion somewhat less when compared to nitrofurantoin. Blood concentrations at therapeutic dosage are usually low. It is highly soluble in urine, to which it may impart a brown color.
Following a dose regimen of 100 mg q.i.d. for 7 days, average urinary drug recoveries (0-24 hours) on day 1 and day 7 were 37.9% and 35.0%.
Unlike many drugs, the presence of food or agents delaying gastric emptying can increase the bioavailability of Nitrofurantoin macrocrystals, presumably by allowing better dissolution in gastric juices.
MICROBIOLOGY
Nitrofurantoin is a nitrofuran antimicrobial agent with activity against certain Gram-positive and Gram-negative bacteria.
Mechanism of Action
The mechanism of the antimicrobial action of nitrofurantoin is unusual among antibacterials.
Nitrofurantoin is reduced by bacterial flavoproteins to reactive intermediates which inactivate or alter bacterial ribosomal proteins and other acromolecules. As a result of such inactivations, the vital biochemical processes of protein synthesis, aerobic energy metabolism, DNA synthesis, RNA synthesis, and cell wall synthesis are inhibited. Nitrofurantoin is bactericidal in urine at therapeutic doses. The broad-based nature of this mode of action may explain the lack of acquired bacterial resistance to nitrofurantoin, as the necessary multiple and simultaneous mutations of the target macromolecules would likely be lethal to the bacteria.
Interactions with Other Antibiotics
Antagonism has been demonstrated in vitro between nitrofurantoin and quinolone antimicrobials. The clinical significance of this finding is unknown.
Development of Resistance
Development of resistance to nitrofurantoin has not been a significant problem since its introduction in 1953. Cross-resistance with antibiotics and sulfonamides has not been observed, and transferable resistance is, at most, a very rare phenomenon.
Nitrofurantoin has been shown to be active against most strains of the following bacteria both in vitro and in clinical infections [see Indications and Usage]:
Aerobic and facultative Gram-positive microorganisms:
Staphylococcus aureus
Enterococci (e.g. Enterococcus faecalis)
Aerobic and facultative Gram-negative microorganisms:
Escherichia coli
NOTE: While nitrofurantoin has excellent activity against Enterococcus faecalis, the majority of Enterococcus faecium isolates are not susceptible to nitorfurantoin.
At least 90 percent of the following microorganisms exhibit an in vitro minimum inhibitory concentration (MIC) less than or equal to the susceptible breakpoint for nitrofurantoin. However, the efficacy of nitrofurantoin in treating clinical infections due to these microorganisms has not been established in adequate and well-controlled trials.
Aerobic and facultative Gram-positive microorganisms:
Coagulase-negative staphylococci (including Staphylococcus epidermidis and Staphylococcus saprophyticus)
Streptococcus agalactiae
Group D streptococci
Viridans group streptococci
Aerobic and facultative Gram-negative microorganisms:
Citrobacter amalonaticus
Citrobacter diversus
Citrobacter freundii
Klebsiella oxytoca
Klebsiella ozaenae
NOTE: Some strains of Enterobacter species and Klebsiella species are resistant to nitrofurantoin.
-
INDICATIONS AND USAGE:
Nitrofurantoin Capsules, USP (macrocrystals) is specifically indicated for the treatment of urinary tract infections when due to susceptible strains of Escherichia coli, enterococci, staphylococcus aureus, and certain susceptible strains of Klebsiella and Enterobacter species.
Nitrofurantoin is not indicated for the treatment of pyelonephritis or perinephric abscesses.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Nitrofurantoin Capsules, USP (macrocrystals) and other antibacterial drugs, Nitrofurantoin Capsules, USP (macrocrystals) should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria.
When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Nitrofurantoins lack the broader tissue distribution of other therapeutic agents approved for urinary tract infections. Consequently, many patients who are treated with Nitrofurantoin Capsules, USP (macrocrystals) are predisposed to persistence or reappearance of bacteriuria. Urine specimens for culture and susceptibility testing should be obtained before and after completion of therapy. If persistence or reappearance of bacteriuria occurs after treatment with Nitrofurantoin Capsules, USP (macrocrystals), other therapeutic agents with broader tissue distribution should be selected. In considering the use of Nitrofurantoin Capsules, USP (macrocrystals), lower eradication rates should be balanced against the increased potential for systemic toxicity and for the development of antimicrobial resistance when agents with broader tissue distribution are utilized.
-
CONTRAINDICATIONS:
Anuria, oliguria, or significant impairment of renal function (creatinine clearance under 60 mL per minute or clinically significant elevated serum creatinine) are contraindications. Treatment of this type of patient carries an increased risk of toxicity because of impaired excretion of the drug.
Because of the possibility of hemolytic anemia due to immature erythrocyte enzyme systems (glutathione instability), the drug is contraindicated in pregnant patients at term (38-42 weeks’gestation), during labor and delivery, or when the onset of labor is imminent. For the same reason, the drug is contraindicated in neonates under one month of age.
Nitrofurantoin Capsules, USP (macrocrystals) is contraindicated in patients with a previous history of cholestatic jaundice/hepatic dysfunction associated with nitrofurantoin.
Nitrofurantoin Capsules, USP (macrocrystals) is also contraindicated in those patients with known hypersensitivity to nitrofurantoin.
-
WARNINGS
Pulmonary reactions:
ACUTE, SUBACUTE, OR CHRONIC PULMONARY REACTIONS HAVE BEEN OBSERVED IN PATIENTS TREATED WITH NITROFURANTOIN. IF THESE REACTIONS OCCUR, NITROFURANTOIN MACROCRYSTALS SHOULD BE DISCONTINUED AND APPROPRIATE MEASURES TAKEN. REPORTS HAVE CITED PULMONARY REACTIONS AS A CONTRIBUTING CAUSE OF DEATH.
CHRONIC PULMONARY REACTIONS (DIFFUSE INTERSTITIAL PNEUMONITIS OR PULMONARY FIBROSIS, OR BOTH) CAN DEVELOP INSIDIOUSLY. THESE REACTIONS OCCUR RARELY AND GENERALLY IN PATIENTS RECEIVING THERAPY FOR SIX MONTHS OR LONGER. CLOSE MONITORING OF THE PULMONARY CONDITION OF PATIENTS RECEIVING LONG-TERM THERAPY IS WARRANTED AND REQUIRES THAT THE BENEFITS OF THERAPY BE WEIGHED AGAINST POTENTIAL RISKS (SEE RESPIRATORY REACTIONS).
Hepatotoxicity:
Hepatic reactions, including hepatitis, cholestatic jaundice, chronic active hepatitis, and hepatic necrosis, occur rarely. Fatalities have been reported. The onset of chronic active hepatitis may be insidious, and patients should be monitored periodically for changes in biochemical tests that would indicate liver injury. If hepatitis occurs, the drug should be withdrawn immediately and appropriate measures should be taken.
Neuropathy:
Peripheral neuropathy, which may become severe or irreversible, has occurred. Fatalities have been reported. Conditions such as renal impairment (creatinine clearance under 60 mL per minute or clinically significant elevated serum creatinine), anemia, diabetes mellitus, electrolyte imbalance, vitamin B deficiency, and debilitating disease may enhance the occurrence of peripheral neuropathy. Patients receiving long-term therapy should be monitored periodically for changes in renal function.
Optic neuritis has been reported rarely in postmarketing experience with nitrofurantoin formulations.
Hemolytic anemia:
Cases of hemolytic anemia of the primaquine-sensitivity type have been induced by nitrofurantoin. Hemolysis appears to be linked to a glucose-6-phosphate dehydrogenase deficiency in the red blood cells of the affected patients. This deficiency is found in 10 percent of Blacks and a small percentage of ethnic groups of Mediterranean and Near-Eastern origin. Hemolysis is an indication for discontinuing nitrofurantoin macrocrystals; hemolysis ceases when the drug is withdrawn.
Clostridium difficile-associated diarrhea:
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including nitrofurantoin, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
-
PRECAUTIONS
Information for Patients
Patients should be advised to take Nitrofurantoin Capsules, USP (macrocrystals) with food to further enhance tolerance and improve drug absorption. Patients should be instructed to complete the full course of therapy; however, they should be advised to contact their physician if any unusual symptoms occur during therapy.
Many patients who cannot tolerate microcrystalline nitrofurantoin are able to take Nitrofurantoin Capsules, USP (macrocrystals) without nausea.
Patients should be advised not to use antacid preparations containing magnesium trisilicate while taking Nitrofurantoin Capsules, USP (macrocrystals).
Patients should be counseled that antibacterial drugs including Nitrofurantoin Capsules, USP (macrocrystals) should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Nitrofurantoin capsule, USP (macrocrystals) is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Nitrofurantoin Capsules, USP (macrocrystals) or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
General:
Prescribing nitrofurantoin macrocrystals in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Drug Interactions:
Antacids containing magnesium trisilicate, when administered concomitantly with nitrofurantoin, reduce both the rate and extent of absorption. The mechanism for this interaction probably is adsorption of nitrofurantoin onto the surface of magnesium trisilicate.
Uricosuric drugs, such as probenecid and sulfinpyrazone, can inhibit renal tubular secretion of nitrofurantoin. The resulting increase in nitrofurantoin serum levels may increase toxicity, and the decreased urinary levels could lessen its efficacy as a urinary tract antibacterial.
Drug/Laboratory Test Interactions:
As a result of the presence of nitrofurantoin, a false-positive reaction for glucose in the urine may occur. This has been observed with Benedict’s and Fehling’s solutions but not with the glucose enzymatic test.
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Nitrofurantoin was not carcinogenic when fed to female Holtzman rats for 44.5 weeks or to female Sprague-Dawley rats for 75 weeks. Two chronic rodent bioassays utilizing male and female Sprague-Dawley rats and two chronic bioassays in Swiss mice and in BDF1 mice revealed no evidence of carcinogenicity.
Nitrofurantoin presented evidence of carcinogenic activity in female B6C3F1 mice as shown by increased incidences of tubular adenomas, benign mixed tumors, and granulosa cell tumors of the ovary. In male F344/N rats, there were increased incidences of uncommon kidney tubular cell neoplasms, osteosarcomas of the bone, and neoplasms of the subcutaneous tissue. In one study involving subcutaneous administration of 75 mg/kg nitrofurantoin to pregnant female mice, lung papillary adenomas of unknown significance were observed in the F1 generation.
Nitrofurantoin has been shown to induce point mutations in certain strains of Salmonella typhimurium and forward mutations in L5178Y mouse lymphoma cells. Nitrofurantoin induced increased numbers of sister chromatid exchanges and chromosomal aberrations in Chinese hamster ovary cells but not in human cells in culture. Results of the sex-linked recessive lethal assay in Drosophila were negative after administration of nitrofurantoin by feeding or by injection. Nitrofurantoin did not induce heritable mutation in the rodent models examined.
The significance of the carcinogenicity and mutagenicity findings relative to the therapeutic use of nitrofurantoin in humans is unknown.
The administration of high doses of nitrofurantoin to rats causes temporary spermatogenic arrest; this is reversible on discontinuing the drug. Doses of 10 mg/kg/day or greater in healthy human males may, in certain unpredictable instances, produce a slight to moderate spermatogenic arrest with a decrease in sperm count.
Pregnancy:
Teratogenic Effects: Pregnancy Category B.
Several reproduction studies have been performed in rabbits and rats at doses up to six times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to nitrofurantoin. In a single published study conducted in mice at 68 times the human dose (based on mg/kg administered to the dam), growth retardation and a low incidence of minor and common malformations were observed. However, at 25 times the human dose, fetal malformations were not observed; the relevance of these findings to humans is uncertain. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nonteratogenic Effects:
Nitrofurantoin has been shown in one published transplacental carcinogenicity study to induce lung papillary adenomas in the F1 generation mice at doses 19 times the human dose on a mg/kg basis. The relationship of this finding to potential human carcinogenesis is presently unknown. Because of the uncertainty regarding the human implications of these animal data, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Nitrofurantoin has been detected in human breast milk in trace amounts. Because of the potential for serious adverse reactions from nitrofurantoin in nursing infants under one month of age, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother (see CONTRAINDICATIONS).
Pediatric Use
Nitrofurantoin macrocrystals is contraindicated in infants below the age of one month (see CONTRAINDICATIONS).
Geriatric Use
Clinical studies of nitrofurantoin macrocrystals did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Spontaneous reports suggest a higher proportion of pulmonary reactions, including fatalities, in elderly patients; these differences appear to be related to the higher proportion of elderly patients receiving long-term nitrofurantoin therapy. As in younger patients, chronic pulmonary reactions generally are observed in patients receiving therapy for six months or longer (see WARNINGS). Spontaneous reports also suggest an increased proportion of severe hepatic reactions, including fatalities, in elderly patients (see WARNINGS).
In general, the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy should be considered when prescribing nitrofurantoin macrocrystals. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Anuria, oliguria, or significant impairment of renal function (creatinine clearance under 60 mL per minute or clinically significant elevated serum creatinine) are contraindications (see CONTRAINDICATIONS). Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
-
ADVERSE REACTIONS
Respiratory:
CHRONIC, SUBACUTE, OR ACUTE PULMONARY HYPERSENSITIVITY REACTIONS MAY OCCUR.
CHRONIC PULMONARY REACTIONS OCCUR GENERALLY IN PATIENTS WHO HAVE RECEIVED CONTINUOUS TREATMENT FOR SIX MONTHS OR LONGER. MALAISE, DYSPNEA ON EXERTION, COUGH, AND ALTERED PULMONARY FUNCTION ARE COMMON MANIFESTATIONS WHICH CAN OCCUR INSIDIOUSLY. RADIOLOGIC AND HISTOLOGIC FINDINGS OF DIFFUSE INTERSTITIAL PNEUMONITIS OR FIBROSIS, OR BOTH, ARE ALSO COMMON MANIFESTATIONS OF THE CHRONIC PULMONARY REACTION. FEVER IS RARELY PROMINENT.
THE SEVERITY OF CHRONIC PULMONARY REACTIONS AND THEIR DEGREE OF RESOLUTION APPEAR TO BE RELATED TO THE DURATION OF THERAPY AFTER THE FIRST CLINICAL SIGNS APPEAR. PULMONARY FUNCTION MAY BE IMPAIRED PERMANENTLY, EVEN AFTER CESSATION OF THERAPY. THE RISK IS GREATER WHEN CHRONIC PULMONARY REACTIONS ARE NOT RECOGNIZED EARLY.
In subacute pulmonary reactions, fever and eosinophilia occur less often than in the acute form. Upon cessation of therapy, recovery may require several months. If the symptoms are not recognized as being drug-related and nitrofurantoin therapy is not stopped, the symptoms may become more severe.
Acute pulmonary reactions are commonly manifested by fever, chills, cough, chest pain, dyspnea, pulmonary infiltration with consolidation or pleural effusion on x-ray, and eosinophilia. Acute reactions usually occur within the first week of treatment and are reversible with cessation of therapy. Resolution often is dramatic (see WARNINGS).
Changes in EKG (e.g., non-specific ST/T wave changes, bundle branch block) have been reported in association with pulmonary reactions.
Cyanosis has been reported rarely.
Hepatic: Hepatic reactions, including hepatitis, cholestatic jaundice, chronic active hepatitis, and hepatic necrosis, occur rarely (see WARNINGS).
Neurologic: Peripheral neuropathy, which may become severe or irreversible, has occurred. Fatalities have been reported. Conditions such as renal impairment (creatinine clearance under 60 mL per minute or clinically significant elevated serum creatinine), anemia, diabetes mellitus, electrolyte imbalance, vitamin B deficiency, and debilitating diseases may increase the possibility of peripheral neuropathy (see WARNINGS).
Asthenia, vertigo, nystagmus, dizziness, headache, and drowsiness also have been reported with the use of nitrofurantoin.
Benign intracranial hypertension (pseudotumor cerebri), confusion, depression, optic neuritis, and psychotic reactions have been reported rarely. Bulging fontanels, as a sign of benign intracranial hypertension in infants, have been reported rarely.
Dermatologic: Exfoliative dermatitis and erythema multiforme (including Stevens-Johnson syndrome) have been reported rarely. Transient alopecia also has been reported.
Allergic: A lupus-like syndrome associated with pulmonary reactions to nitrofurantoin has been reported. Also, angioedema; maculopapular, erythematous, or eczematous eruptions; pruritus; urticaria; anaphylaxis; arthralgia; myalgia; drug fever; chills; and vasculitis (sometimes associated with pulmonary reactions) have been reported. Hypersensitivity reactions represent the most frequent spontaneously-reported adverse events in worldwide post-marketing experience with nitrofurantoin formulations.
Gastrointestinal: Nausea, emesis, and anorexia occur most often. Abdominal pain and diarrhea are less common gastrointestinal reactions. These dose-related reactions can be minimized by reduction of dosage. Sialadenitis and pancreatitis have been reported. There have been sporadic reports of pseudomembranous colitis with the use of nitrofurantoin. The onset of pseudomembranous colitis symptoms may occur during or after antimicrobial treatment (see WARNINGS).
Hematologic: Cyanosis secondary to methemoglobinemia has been reported rarely.
Miscellaneous: As with other antimicrobial agents, superinfections caused by resistant organisms, e.g., Pseudomonas species or Candida species, can occur.
Laboratory Adverse Events: The following laboratory adverse events have been reported with the use of nitrofurantoin: increased AST (SGOT), increased ALT (SGPT), decreased hemoglobin, increased serum phosphorus, eosinophilia, glucose-6-phosphate dehydrogenase deficiency anemia (see WARNINGS), agranulocytosis, leukopenia, granulocytopenia, hemolytic anemia, thrombocytopenia, megaloblastic anemia. In most cases, these hematologic abnormalities resolved following cessation of therapy. Aplastic anemia has been reported rarely.
-
OVERDOSAGE:
Occasional incidents of acute overdosage of nitrofurantoin macrocrystals have not resulted in any specific symptoms other than vomiting. Induction of emesis is recommended. There is no specific antidote, but a high fluid intake should be maintained to promote urinary excretion of the drug. It is dialyzable.
-
DOSAGE AND ADMINISTRATION:
Nitrofurantoin Capsules, USP (macrocrystals) should be given with food to improve drug absorption and, in some patients, tolerance.
Adults: 50-100 mg four times a day — the lower dosage level is recommended for uncomplicated urinary tract infections.
Pediatric Patients: 5-7 mg/kg of body weight per 24 hours, given in four divided doses (contraindicated under one month of age).
Therapy should be continued for one week or for at least 3 days after sterility of the urine is obtained. Continued infection indicates the need for re-evaluation.
For long-term suppressive therapy in adults, a reduction of dosage to 50-100 mg at bedtime may be adequate. For long-term suppressive therapy in pediatric patients, doses as low as 1 mg/kg per 24 hours, given in a single dose or in two divided doses, may be adequate. SEE WARNINGS SECTION REGARDING RISKS ASSOCIATED WITH LONG-TERM THERAPY.
-
HOW SUPPLIED
Nitrofurantoin Capsules, USP (Macrocrystals), 25 mg are size “4” white opaque hard gelatin capsule, axially printed “231” in black ink on both cap and body containing cream to yellow colored powder available as follows:
Nitrofurantoin Capsules, USP (Macrocrystals), 50 mg are size “3” light brown hard gelatin capsule, axially printed “232” in black ink on both cap and body containing cream to yellow colored powder available as follows:
Nitrofurantoin Capsules, USP (Macrocrystals), 100 mg are size “2” grey opaque hard gelatin capsule, axially printed “233” in black ink on both cap and body containing cream to yellow colored powder available as follows:
55700-893-14
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature].
Protect from light and moisture.
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-406-7984 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512
Manufactured by:
Sidmak Laboratories (India) Pvt. Ltd.
Plot No. 20, Pharmacity, Selaqui Industrial Area,
Dehradun - 248 197
Uttarakhand, India
Rev. 11/18
M. L. No. 38/UA/2007
P2081002
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NITROFURANTION
nitrofurantion capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:55700-893(NDC:57664-233) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NITROFURANTOIN (UNII: 927AH8112L) (NITROFURANTOIN - UNII:927AH8112L) NITROFURANTOIN 100 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) SHELLAC (UNII: 46N107B71O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Product Characteristics Color gray (Opaque) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code 233 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:55700-893-14 14 in 1 BOTTLE; Type 0: Not a Combination Product 01/04/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA201722 01/04/2021 Labeler - Quality Care Products, LLC (831276758) Establishment Name Address ID/FEI Business Operations Quality Care Products, LLC 831276758 repack(55700-893)