Label: YES TO TOMATOES ACNE FIGHTING SPOT TREATMENT- salicylic acid liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 69840-014-05 - Packager: Yes To, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 29, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active Ingredient
- Purpose
- Uses
- Warnings
-
Directions
- clean the skin thoroughly before applying this product
- cover the entire affected area with a thin layer using the rollerball applicator one to three times daily
- because excessive drying of the skin may occur, start with one application daily, then gradually increase to two or three times daily if needed or as directed by a doctor
- if bothersome dryness or peeling occurs, reduce application to once a day or every other day.
-
Inactive Ingredients
Aloe Barbadensis Leaf Juice, Water (Aqua), Hamamelis Virginiana (Witch Hazel) Water, Coco-Caprylate, Acacia Senegal Gum, Octyldodecanol, Xanthan Gum, Propanediol, Dicaprylyl Ether, C14-22 Alcohols, Arachidyl Alcohol, Biosaccharide Gum-1, Tocopheryl Acetate, Solanum Lycopersicum (Tomato) Extract, Charcoal Powder, Zingiber Officinale (Ginger) Root Juice, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Glycerin, Sodium Hydroxide, Ethylhexyglycerin, Allantoin, Bisabolol, Behenyl Alcohol, C12-20 Alkyl Glucoside, Arachidyl Glucoside, Phenoxyethanol, Fragrance (Parfum), Benzyl Benzoate, Limonene, Butylphenyl Methylpropional, Coumarin.
- Questions?
-
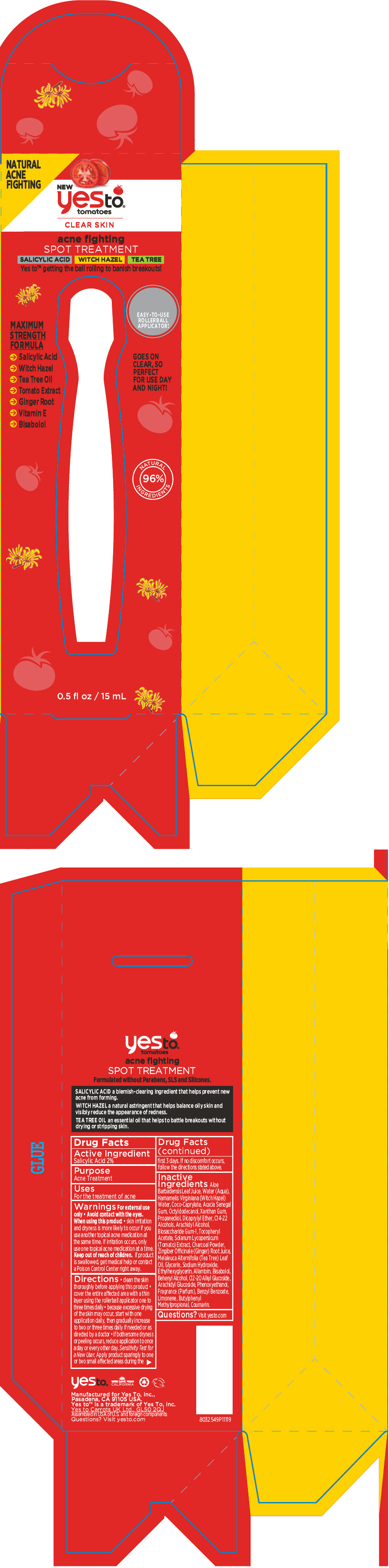
PRINCIPAL DISPLAY PANEL - 15 mL Bottle Box
NATURAL
ACNE
FIGHTINGNEW
yesto®
tomatoesCLEAR SKIN
acne fighting
SPOT TREATMENTSALICYLIC ACID
WITCH HAZEL
TEA TREEYes to™ getting the ball rolling to banish breakouts!
MAXIMUM
STRENGTH
FORMULA- →
- Salicylic Acid
- →
- Witch Hazel
- →
- Tea Tree Oil
- →
- Tomato Extract
- →
- Ginger Root
- →
- Vitamin E
- →
- Bisabolol
EASY-TO-USE
ROLLERBALL
APPLICATOR!GOES ON
CLEAR, SO
PERFECT
FOR USE DAY
AND NIGHT!NATURAL
96%
INGREDIENTS0.5 fl oz / 15 mL
-
INGREDIENTS AND APPEARANCE
YES TO TOMATOES ACNE FIGHTING SPOT TREATMENT
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69840-014 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) HAMAMELIS VIRGINIANA TOP WATER (UNII: NT00Y05A2V) PROPANEDIOL (UNII: 5965N8W85T) ALLANTOIN (UNII: 344S277G0Z) GLYCERIN (UNII: PDC6A3C0OX) ACTIVATED CHARCOAL (UNII: 2P3VWU3H10) SOLANUM LYCOPERSICUM (UNII: 0243Q4990L) TEA TREE OIL (UNII: VIF565UC2G) COCO-CAPRYLATE/CAPRATE (UNII: 8D9H4QU99H) ACACIA (UNII: 5C5403N26O) OCTYLDODECANOL (UNII: 461N1O614Y) XANTHAN GUM (UNII: TTV12P4NEE) DICAPRYLYL ETHER (UNII: 77JZM5516Z) C14-22 ALCOHOLS (UNII: B1K89384RJ) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) BIOSACCHARIDE GUM-1 (UNII: BB4PU4V09H) PHENOXYETHANOL (UNII: HIE492ZZ3T) DOCOSANOL (UNII: 9G1OE216XY) C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) SODIUM HYDROXIDE (UNII: 55X04QC32I) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) .ALPHA.-BISABOLOL, (+/-)- (UNII: 36HQN158VC) GINGER (UNII: C5529G5JPQ) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69840-014-05 1 in 1 BOX 06/01/2019 1 15 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part333D 06/01/2019 Labeler - Yes To, Inc. (788689680)