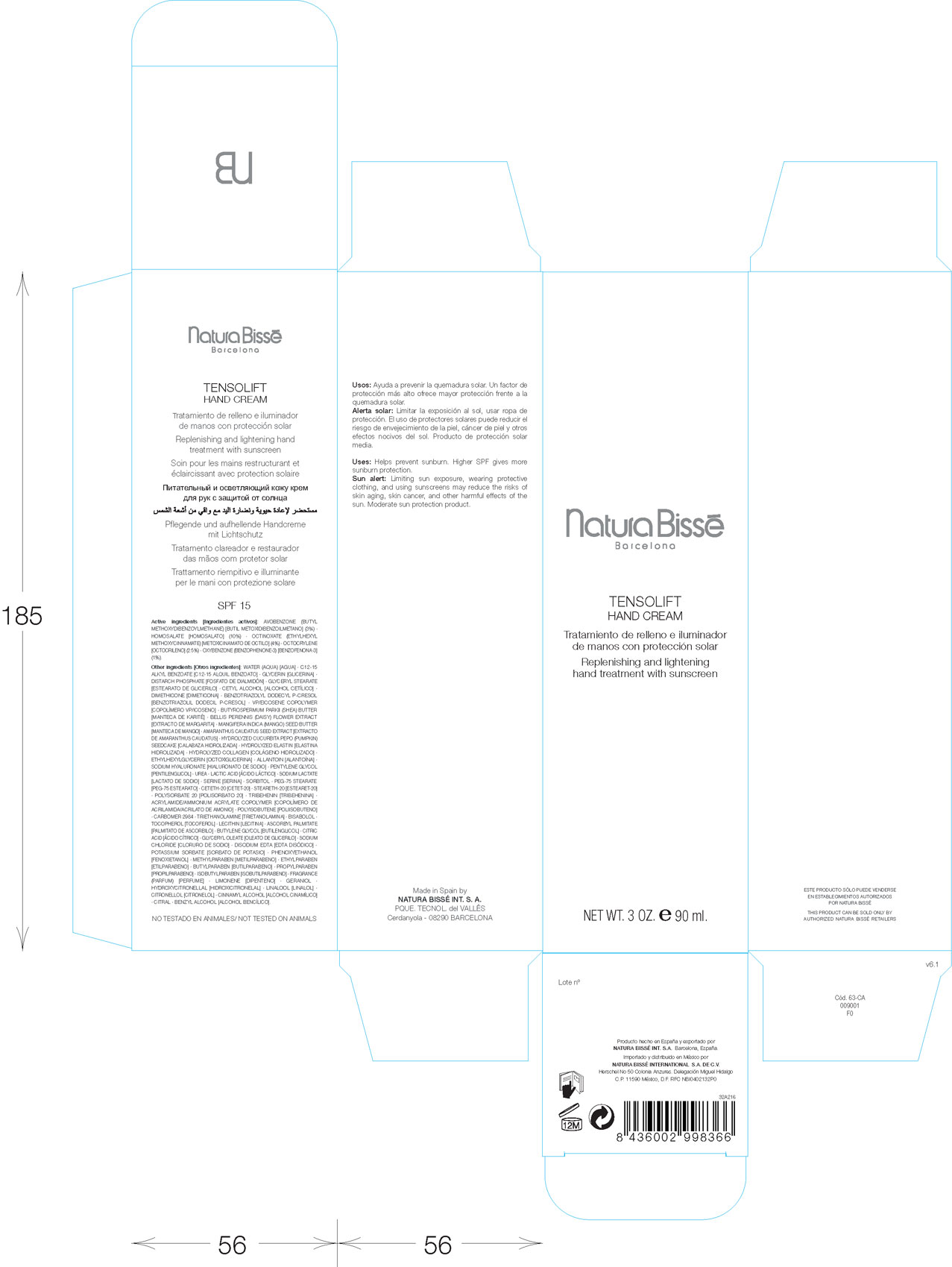
Label: TENSOLIFT- avobenzone, homosalate, octinoxate, octyocrelene, oxybenzone cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 63730-216-01, 63730-216-02, 63730-216-03, 63730-216-04, view more63730-216-05 - Packager: Natura Bisse International, S.A.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 8, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Other Ingredients: Water (Aqua). C12-15 Alkyl Benzoate. Glycerin. Distarch Phsphate. Glyceryl Stearate. Cetyl Alcohol. Dimethicone. Benzotriazolyl Dodecyl P-Cresol. VP/Eicosene Copolymer. Butyrospermum Parkii (SHEA) Butter. Amaranthus Caudatus Seed Extract. Hydrolyzed Cucurbita Pepo (Pumkin) Seedcake. Hydrolyzed Elastin. Hydrolyzed Collagen. Ethylhexylglycerin. Allantoin. Sodium Hyaluronate. Pentylene Glycol. Urea. Lactic Acid. Sodium Lactate. Serine. Sorbitol. PEG-75 Stearate. Ceteth-20. Steareth-20. Polysorbate 20. Tribehenin. Acrylamide/Ammoniu, Acrylate Copolymer. Polyisobutene. Carbomer 2984. Trietholamine. Bisabolol. Tocopherol. Lecithin. Ascorbyl Palmitate. Butylene Glycol. Citric Acid. Glyceryl Oleate. Sodium Chloride. Disodium Edta. Potassium Sorbate. Phynoxyethanol. Methylparaben. Ethylparaben. butylparaben. Propylparaben. Isobutylparaben. Fragrance (Parfum). Limonene. Geraniol. Hydroxycitronellal. Linalool. Citronellel. Cinnamyl Alcohol. Citral. Benzyl Alcohol.
- PURPOSE
-
PRINCIPAL DISPLAY PANEL
Natura Bisse
Barcelona
Tensolift Hand Cream
Replenishing and lightening hand treatment with sunscreen
Net Wt. 3 Oz. 90 ml.
Made in Spain by
Natura Bisse Int. S.A.
Cerdanyola - 08290 Barcelona
Herschel No 50 Colonia Anzures. Delegaciun Miguel Hidalgo.
C.P. 11590 Mexico, D.F. RFC NBI0402132P0
Not tested on animals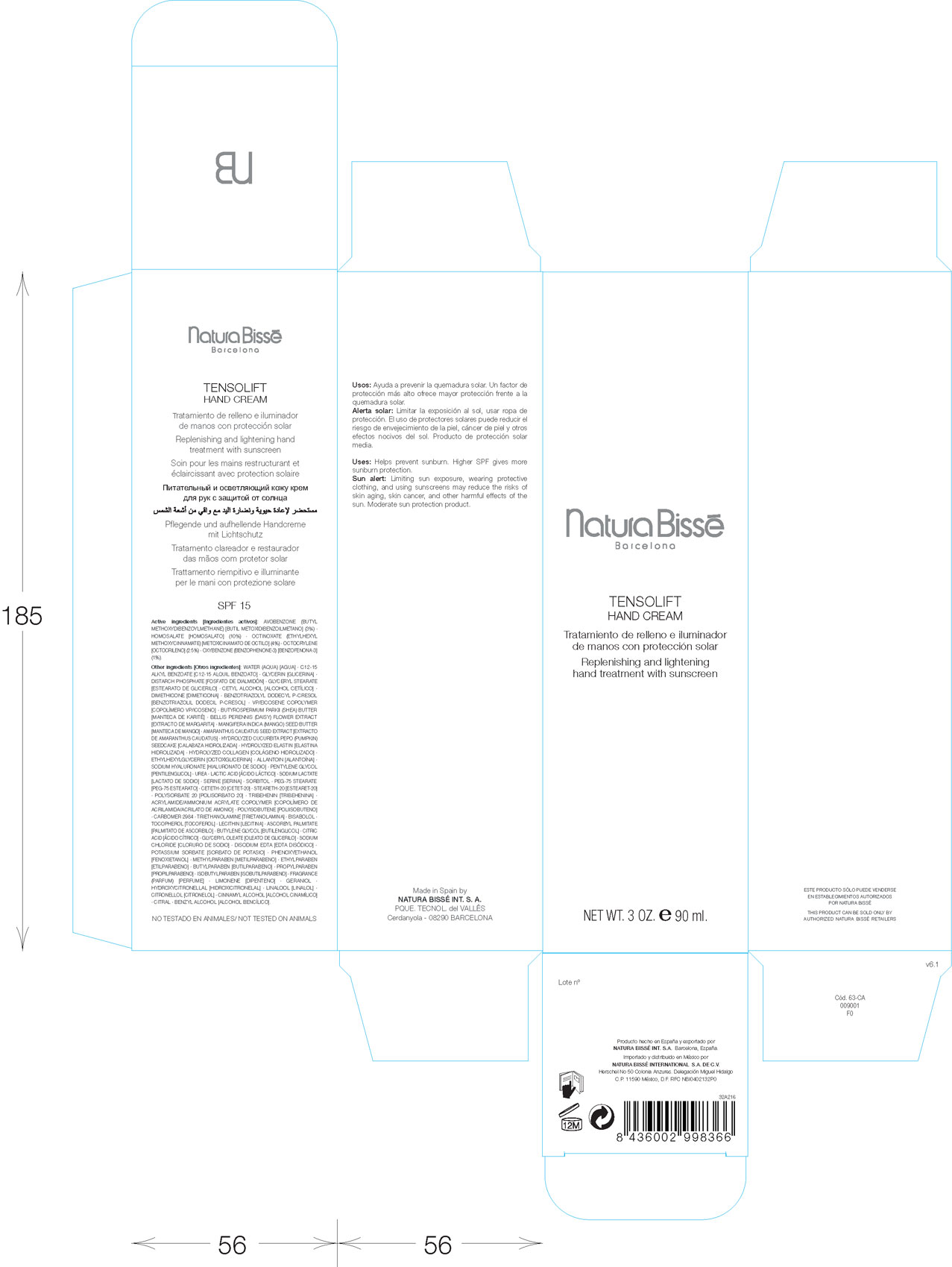
-
INGREDIENTS AND APPEARANCE
TENSOLIFT
avobenzone, homosalate, octinoxate, octyocrelene, oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63730-216 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.76 g in 90 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 8.76 mL in 90 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 3.65 mL in 90 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.19 mL in 90 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 921 mg in 90 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) C12-15 ALKYL BENZOATE (UNII: A9EJ3J61HQ) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETYL ALCOHOL (UNII: 936JST6JCN) DIMETHICONE (UNII: 92RU3N3Y1O) BELLIS PERENNIS (UNII: 2HU33I03UY) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ALLANTOIN (UNII: 344S277G0Z) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PENTYLENE GLYCOL (UNII: 50C1307PZG) UREA (UNII: 8W8T17847W) LACTIC ACID (UNII: 33X04XA5AT) SODIUM LACTATE (UNII: TU7HW0W0QT) SERINE (UNII: 452VLY9402) SORBITOL (UNII: 506T60A25R) CETETH-20 (UNII: I835H2IHHX) STEARETH-20 (UNII: L0Q8IK9E08) POLYSORBATE 20 (UNII: 7T1F30V5YH) TRIBEHENIN (UNII: 8OC9U7TQZ0) TROLAMINE (UNII: 9O3K93S3TK) LEVOMENOL (UNII: 24WE03BX2T) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) ASCORBYL PALMITATE (UNII: QN83US2B0N) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERYL MONOOLEATE (UNII: 4PC054V79P) SODIUM CHLORIDE (UNII: 451W47IQ8X) EDETATE DISODIUM (UNII: 7FLD91C86K) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) ETHYLPARABEN (UNII: 14255EXE39) BUTYLPARABEN (UNII: 3QPI1U3FV8) PROPYLPARABEN (UNII: Z8IX2SC1OH) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) GERANIOL (UNII: L837108USY) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LINALOOL, DL- (UNII: D81QY6I88E) CINNAMYL ALCOHOL (UNII: SS8YOP444F) CITRAL (UNII: T7EU0O9VPP) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63730-216-01 90 mL in 1 TUBE 2 NDC:63730-216-02 2 mL in 1 TUBE 3 NDC:63730-216-03 20 mL in 1 PACKAGE 4 NDC:63730-216-04 200 mL in 1 PACKAGE 5 NDC:63730-216-05 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 06/07/2010 Labeler - Natura Bisse International, S.A. (464431576) Registrant - Natura Bisse International, S.A. (464431576) Establishment Name Address ID/FEI Business Operations Natura Bisse International, S.A. 464431576 manufacture