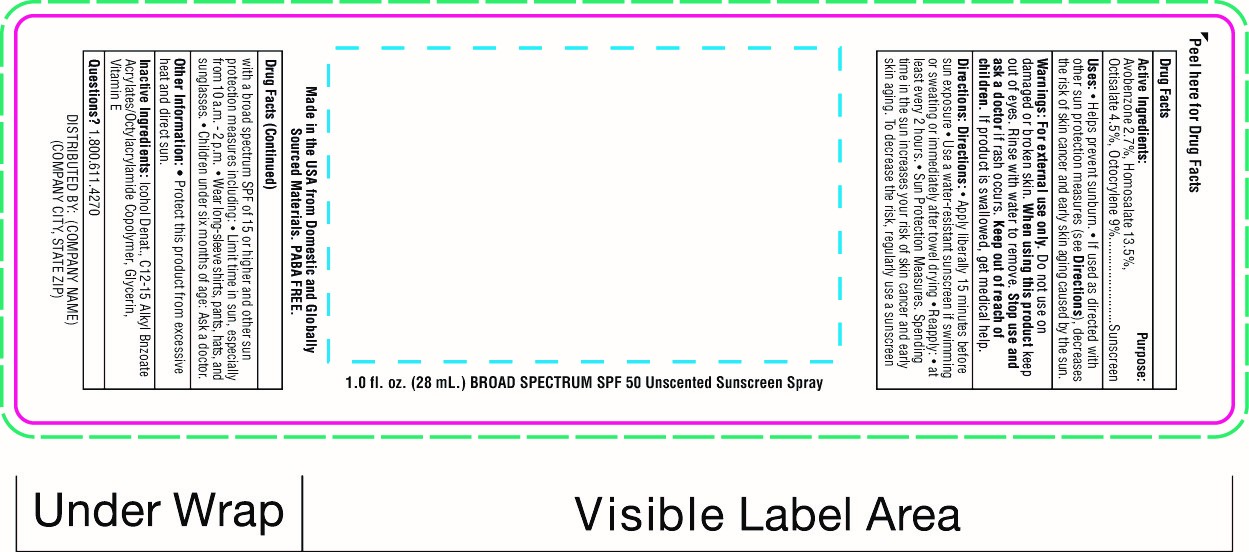
Label: SNUGZ SPF 50 SUNSCREEN SPRY- spf 50 sunscreen spray spray
- NDC Code(s): 76309-525-10, 76309-525-71
- Packager: SnugZ USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPF 50 Sunscreen Spray
- SPF 50 Sunscreen Spray
- SPF 50 Sunscreen Spray
- SPF 50 Sunscreen Spray
- SPF 50 Sunscreen Spray
- SPF 50 Sunscreen Spray
- ZSUNPLS - SPF 50 Sunscreen Spray
-
INGREDIENTS AND APPEARANCE
SNUGZ SPF 50 SUNSCREEN SPRY
spf 50 sunscreen spray sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76309-525 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 1.4 g in 28 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2.8 g in 28 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 4.2 g in 28 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 0.84 g in 28 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76309-525-10 10 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 06/01/2019 2 NDC:76309-525-71 28 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 06/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2019 Labeler - SnugZ USA (615959228) Registrant - SnugZ/USA, LLC (615959228) Establishment Name Address ID/FEI Business Operations SnugZ USA 615959228 manufacture(76309-525)