Label: TARPEYO- budesonide capsule, delayed release
- NDC Code(s): 81749-004-01
- Packager: Calliditas Therapeutics AB
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated June 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TARPEYO safely and effectively. See full prescribing information for TARPEYO.
TARPEYO (budesonide) delayed release capsules, for oral use
Initial U.S. Approval: 1997INDICATIONS AND USAGE
TARPEYO is a corticosteroid indicated to reduce the loss of kidney function in adults with primary immunoglobulin A nephropathy (IgAN) who are at risk for disease progression. (1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Delayed release capsules: 4 mg (3)
CONTRAINDICATIONS
- Hypersensitivity to budesonide or any of the ingredients in TARPEYO. (4)
WARNINGS AND PRECAUTIONS
- Hypercorticism and Adrenal Axis Suppression: Follow general warnings concerning corticosteroids, patients with hepatic impairment may be at increased risk. Taper upon discontinuation. (2, 5.1, 8.6, 12.3)
- Immunosuppression and Increased Risk of Infection: Avoid use in patients with active or quiescent tuberculosis infection, untreated fungal, bacterial, systemic viral or parasitic infections, or ocular herpes simplex. May affect vaccine efficacy. (5.2)
- Other Corticosteroid Effects: Monitor patients with concomitant conditions where corticosteroids may have unwanted effects (e.g., hypertension, diabetes mellitus). (5.3)
ADVERSE REACTIONS
Most common adverse reactions ( ≥5%) are peripheral edema, hypertension, muscle spasms, acne, headache, upper respiratory tract infection, face edema, weight increased, dyspepsia, dermatitis, arthralgia, white blood cell count increased. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Calliditas Therapeutics at 1-844-IGA-0011 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Potent CYP3A4 Inhibitors (e.g. ketoconazole, grapefruit juice): Can increase systemic budesonide concentrations: avoid concomitant use. (7.1)
USE IN SPECIFIC POPULATIONS
Lactation: Routine monitoring of linear growth in infants is recommended with chronic use of budesonide in the nursing mother. (8.2).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypercorticism and Adrenal Axis Suppression
5.2 Immunosuppression and Increased Risk of Infection
5.3 Other Corticosteroid Effects
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Interaction with CYP3A4 Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Treatment of IgAN
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
The recommended duration of therapy is 9 months, with a dosage of 16 mg administered orally once daily [see Clinical Studies (14.1)]. When discontinuing therapy, reduce the dosage to 8 mg once daily for the last 2 weeks of therapy [see Warnings and Precautions (5.1)].
The delayed release capsules should be swallowed whole in the morning, at least 1 hour before a meal. Do not open, crush or chew.
If a dose is missed, take the prescribed dose at the next scheduled time. Do not double the next dose.
Safety and efficacy of treatment with subsequent courses of TARPEYO have not been established.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypercorticism and Adrenal Axis Suppression
When corticosteroids are used chronically, systemic effects such as hypercorticism and adrenal suppression may occur. Corticosteroids can reduce the response of the hypothalamus-pituitary-adrenal (HPA) axis to stress. In situations where patients are subject to surgery or other stress situations, supplementation with a systemic corticosteroid is recommended. When discontinuing therapy [see Dosing and Administration (2)] or switching between corticosteroids, monitor for signs of adrenal axis suppression.
Patients with moderate to severe hepatic impairment (Child-Pugh Class B and C respectively) could be at an increased risk of hypercorticism and adrenal axis suppression due to an increased systemic exposure of oral budesonide. Avoid use in patients with severe hepatic impairment (Child-Pugh Class C). Monitor for increased signs and/or symptoms of hypercorticism in patients with moderate hepatic impairment (Child-Pugh Class B) [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
5.2 Immunosuppression and Increased Risk of Infection
Corticosteroids, including TARPEYO, suppress the immune system and increase the risk of infection with any pathogen, including viral, bacterial, fungal, protozoan, or helminthic pathogens. Corticosteroids can:
- Reduce resistance to new infections
- Exacerbate existing infections
- Increase the risk of disseminated infections
- Increase the risk of reactivation or exacerbation of latent infections
- Mask some signs of infection
Corticosteroid-associated infections can be mild but can be severe and at times fatal. The rate of infectious complications increases with increasing corticosteroid dosages.
Monitor for the development of infection and consider TARPEYO withdrawal as needed.
Tuberculosis
If TARPEYO is used to treat a condition in patients with latent tuberculosis or tuberculin reactivity, reactivation of tuberculosis may occur. In patients with latent tuberculosis or tuberculin reactivity TARPEYO should be discontinued.
Varicella Zoster and Measles Viral Infections
Varicella and measles can have a serious or even fatal course in non-immune patients taking corticosteroids, including TARPEYO. In corticosteroid-treated patients who have not had these diseases or are non-immune, particular care should be taken to avoid exposure to varicella and measles:
- If a TARPEYO-treated patient is exposed to varicella, prophylaxis with varicella zoster immune globulin may be indicated. If varicella develops, treatment with antiviral agents may be considered.
- If a TARPEYO-treated patient is exposed to measles, prophylaxis with immunoglobulin may be indicated.
Hepatitis B Virus Reactivation
Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers treated with immunosuppressive dosages of corticosteroids, including TARPEYO. Reactivation can also occur infrequently in corticosteroid-treated patients who appear to have resolved hepatitis B infection.
Screen patients for hepatitis B infection before initiating immunosuppressive treatment with TARPEYO. For patients who show evidence of hepatitis B infection, recommend consultation with physicians with expertise in managing hepatitis B regarding monitoring and consideration for hepatitis B antiviral therapy.
Fungal Infections
Corticosteroids, including TARPEYO, may exacerbate systemic fungal infections; therefore, avoid TARPEYO use in the presence of such infections.
Amebiasis
Corticosteroids, including TARPEYO, may activate latent amebiasis. Therefore, it is recommended that latent amebiasis or active amebiasis be ruled out before initiating TARPEYO in patients who have spent time in the tropics or patients with unexplained diarrhea.
Strongyloides Infestation
Corticosteroids, including TARPEYO, should be discontinued in patients with known or suspected Strongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
Ocular Herpes Simplex Virus Infection
Corticosteroids, including TARPEYO, may exacerbate ocular herpes simplex virus infections; therefore, avoid TARPEYO use in the presence of such infections.
5.3 Other Corticosteroid Effects
TARPEYO is a systemically available corticosteroid and is expected to cause related adverse reactions. Monitor patients with hypertension, prediabetes, diabetes mellitus, osteoporosis, peptic ulcer, glaucoma or cataracts, or with a family history of diabetes or glaucoma, or with any other condition where corticosteroids may have unwanted effects.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypercorticism and adrenal axis suppression [see Warnings and Precautions (5.1)]
- Risks of immunosuppression [see Warnings and Precautions (5.2)]
- Other corticosteroid effects [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of TARPEYO was evaluated in 389 patients in the randomized, double-blind, placebo-controlled study, NefIgArd (NCT: 03643965, Phase 3 clinical study in adults with primary IgAN). The data below reflect TARPEYO exposure in 195 patients with a median duration of 41 weeks, compared with comparable exposure to placebo in 194 patients.
The most common adverse reactions, reported in greater than or equal to 5% of TARPEYO-treated patients and greater than or equal to 2% higher than placebo, in the 9-month treatment period are listed in Table 1.
Most adverse reactions that occurred at a greater incidence for TARPEYO compared to placebo were consistent with hypercortisolism and reversible, resolving within 3 months after discontinuation.
Table 1: Reported adverse reactions occurring in greater than or equal to 5% of TARPEYO treated patients, and greater than or equal to 2% higher than Placebo Adverse Reaction TARPEYO 16 mg
(N=195)Placebo
(N=194)n (%) n (%) Peripheral edema 33 (17) 10 (5) Hypertension 23 (12) 6 (3) Muscle spasms 23 (12) 8 (4) Acne 22 (11) 2 (1) Headache 19 (10) 14 (7) Upper respiratory tract infection 16 (8) 12 (6) Face edema 15 (8) 1 (0.5) Weight increased 13 (7) 6 (3) Dyspepsia 13 (7) 4 (2) Dermatitis 12 (6) 2 (1) Arthralgia 12 (6) 4 (2) White blood cell count increased 11 (6) 1 (0.5) -
7 DRUG INTERACTIONS
7.1 Interaction with CYP3A4 Inhibitors
Budesonide is a substrate for CYP3A4. Avoid use with potent CYP3A4 inhibitors; e.g. ketoconazole, itraconazole, ritonavir, indinavir, saquinavir, erythromycin, and cyclosporine [see Clinical Pharmacology (12.3)].
Avoid ingestion of grapefruit juice with TARPEYO. Intake of grapefruit juice, which inhibits CYP3A4 activity, can increase the systemic exposure to budesonide [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The available data from published case series, epidemiological studies and reviews with oral budesonide use in pregnant women have not identified a drug-associated risk of major birth defects, miscarriage or other adverse maternal or fetal outcomes. There are risks to the mother and fetus associated with IgA Nephropathy. Infants exposed to in utero corticosteroids, including budesonide, are at risk for hypoadrenalism (see Clinical Considerations). In animal reproduction studies with pregnant rats and rabbits, administration of subcutaneous budesonide during organogenesis at doses approximately 0.3 times or 0.03 times, respectively, the maximum recommended human dose (MRHD), resulted in increased fetal loss, decreased pup weights, and skeletal abnormalities. Maternal toxicity was observed in both rats and rabbits at these dose levels (see Data).
The estimated background risk of major birth defects and miscarriage of the indicated population is unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
IgA nephropathy in pregnancy is associated with adverse maternal outcomes, including increased rates of cesarean section, pregnancy-induced hypertension, pre-eclampsia and preterm delivery, and adverse fetal/neonatal outcomes, including stillbirth and low birth weight.
Fetal/Neonatal Adverse Reactions
Hypoadrenalism may occur in infants born to mothers receiving corticosteroids during pregnancy. Infants should be carefully observed for signs of hypoadrenalism, such as poor feeding, irritability, weakness, and vomiting, and managed accordingly [see Warnings and Precautions (5.1)].
Data
Animal Data
Budesonide was teratogenic and embryo-lethal in rabbits and rats.
In an embryo-fetal development study in pregnant rats dosed subcutaneously with budesonide during the period of organogenesis on gestation days 6 to 15 there were effects on fetal development and survival at subcutaneous doses up to approximately 500 mcg/kg in rats (approximately 0.3 times the maximum recommended human dose (MRHD) on a body surface area basis).
In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis on gestation days 6 to 18, there was an increase in maternal abortion, and effects on fetal development and reduction in litter weights at subcutaneous doses from approximately 25 mcg/kg (approximately 0.03 times the MRHD on a body surface area basis).
Maternal toxicity, including reduction in body weight gain, was observed at subcutaneous doses of 5 mcg/kg in rabbits (approximately 0.006 times the maximum recommended human dose on a body surface area basis) and 500 mcg/kg in rats (approximately 0.3 times the maximum recommended human dose on a body surface area basis).
In a peri- and post-natal development study, subcutaneous treatment of pregnant rats with budesonide during the period from Day 15 post coitum to Day 21 post partum, budesonide had no effects on delivery, but did have an effect on growth and development of offspring. In addition, offspring survival was reduced and surviving offspring had decreased mean body weights at birth and during lactation at exposures ≥ 0.012 times the MRHD (on a mg/m2 basis at maternal subcutaneous doses of 20 mcg/kg/day and higher). These findings occurred in the presence of maternal toxicity.
8.2 Lactation
Risk Summary
Breastfeeding is not expected to result in significant exposure of the infant to TARPEYO. Lactation studies have not been conducted with oral budesonide, including TARPEYO, and no information is available on the effects of the drug on the breastfed infant or the effects of the drug on milk production. One published study reports that budesonide is present in human milk following maternal inhalation of budesonide (see Data). Routine monitoring of linear growth in infants is recommended with chronic use of budesonide in the nursing mother. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for TARPEYO and any potential adverse effects on the breastfed infant from TARPEYO, or from the underlying maternal condition.
Data
One published study reports that budesonide is present in human milk following maternal inhalation of budesonide, which resulted in infant doses approximately 0.3% to 1% of the maternal weight-adjusted dosage and a milk to plasma ratio was approximately 0.5. Budesonide was not detected in plasma, and no adverse events were noted in the breastfed infants following maternal use of inhaled budesonide.
Assuming a daily average milk intake of about 150 mL/kg/day and a milk to plasma ratio of 0.5, the estimated oral dose of budesonide for a 5 kg infant is expected to be less than 2 mcg/day for a maternal dose of 16 mg TARPEYO. Assuming 100% bio-availability in the infant this is about 0.1% of the maternal dose and about 3% of the highest inhaled dose used clinically for asthma in infants.
8.4 Pediatric Use
The safety and efficacy of TARPEYO in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of TARPEYO did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Hepatic Impairment
Patients with moderate to severe hepatic impairment (Child-Pugh Class B and C, respectively) could be at an increased risk of hypercorticism and adrenal axis suppression due to an increased systemic exposure to budesonide [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.3)]. Avoid use in patients with severe hepatic impairments (Child-Pugh Class C). Monitor for increased signs and/or symptoms of hypercorticism in patients with moderate hepatic impairment (Child-Pugh Class B).
- 10 OVERDOSAGE
-
11 DESCRIPTION
TARPEYO (budesonide) delayed release capsules, for oral administration, contain budesonide, a synthetic corticosteroid, as the active ingredient. Budesonide is designated chemically as 16α, 17α-[(1RS)-Butylidenebis(oxy)]-11β, 21-dihydroxypregna-1,4-diene-3,20-dione.
Budesonide is provided as a mixture of two epimers (22R and 22S). The empirical formula of budesonide is C25H34O6 and its molecular weight is 430.5. Its structural formula is:
Budesonide is a white to off-white, tasteless, odorless powder that is practically insoluble in water, sparingly soluble in alcohol, and freely soluble in chloroform.
The beads in each capsule contain the following inactive ingredients: sugar spheres (sucrose and starch), hypromellose, polyethylene glycol, citric acid monohydrate, ethyl cellulose, medium chain triglycerides and oleic acid. The capsule shells contain hypromellose and titanium oxide (E171); and the printing ink on the capsules contain shellac, propylene glycol and black iron oxide (E172). The enteric coating on the capsules contain: methacrylic acid and methacrylate copolymer, talc and dibutyl sebacate.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Budesonide is a corticosteroid with potent glucocorticoid activity and weak mineralocorticoid activity that undergoes substantial first pass metabolism. Mucosal B-cells present in the ileum, including the Peyer's patches, express glucocorticoid receptors and are responsible for the production of galactose-deficient IgA1 antibodies (Gd-Ag1) causing IgA nephropathy. Through their anti-inflammatory and immunosuppressive effects at the glucocorticoid receptor, corticosteroids can modulate B-cell numbers and activity. It has not been established to what extent TARPEYO's efficacy is mediated via local effects in the ileum vs systemic effects.
12.2 Pharmacodynamics
Treatment with corticosteroids, including TARPEYO, is associated with a suppression of endogenous cortisol concentrations and an impairment of the hypothalamus-pituitary-adrenal (HPA) axis function.
12.3 Pharmacokinetics
Absorption
Following single oral administration of TARPEYO 16 mg to healthy subjects, the average geometric mean Cmax (CV%) was 4.4 ng/mL (58.3), and AUC0-24 was 24.1 h*ng/mL (49.7). Median Tlag (min, max) was 3.1 h (0, 6) while median Tmax (min, max) was 5.1 h (4.5, 10).
Distribution
Approximately 85 to 90% of budesonide binds to plasma proteins in blood over the concentration range of 0.43 to 99 ng/mL. The volume of distribution at steady state reported in the literature is 3 to 4 L/kg.
Metabolism
Budesonide is metabolized by the liver (and to lesser extent the gut), primarily by oxidative pathways via CYP3A4 to two main metabolites, 16α-hydroxyprednisolone and 6β-hydroxybudesonide, which have less than 1% of the corticosteroid activity of budesonide.
Elimination
Budesonide had a high plasma clearance, 0.9 to 1.8 L/min in healthy adults, which is close to the estimated liver blood flow, and, accordingly, suggests that budesonide is a high hepatic clearance drug.
Following single oral administration of TARPEYO 16 mg to healthy subjects, the elimination half-life (t½) for TARPEYO ranged from 5.0 to 6.8 hours.
Excretion
Budesonide was excreted in urine and feces in the form of metabolites. After oral as well as intravenous administration of micronized [3H]-budesonide, approximately 60% of the recovered radioactivity was found in urine. The major metabolites, including 16α-hydroxyprednisolone and 6β-hydroxybudesonide, are mainly renally excreted, intact or in conjugated forms. No unchanged budesonide was detected in urine.
Specific Populations
Age, race, and body weight
The effect of age, race, and body weight on the pharmacokinetics of TARPEYO has not been established.
Sex
Of the 143 healthy volunteers included in the Phase 1 studies, 29% were female. Pharmacokinetics of budesonide was similar between males and females.
Hepatic Impairment
Subjects with moderate hepatic impairment (Child-Pugh class B) had 3.5 times the budesonide AUC compared with healthy volunteers while subjects with mild hepatic impairment (Child-Pugh class A) had approximately 1.4 times the budesonide AUC compared with healthy volunteers.
Patients with severe hepatic impairment (Child-Pugh Class C) have not been studied.
Drug Interaction Studies
Budesonide is metabolized via CYP3A4. Potent inhibitors of CYP3A4 can increase plasma levels of budesonide.
Thus, clinically relevant drug interactions with potent CYP3A4 inhibitors, such as ketoconazole, itraconazole, ritonavir, indinavir, saquinavir, erythromycin, cyclosporine, and grapefruit juice, are to be expected. Conversely, induction of CYP3A4 potentially could result in the lowering of budesonide plasma concentrations.
Effects of Other Drugs on Budesonide
Ketoconazole
In an open, non-randomized, cross-over study, 6 healthy subjects were given budesonide 10 mg as a single dose, either alone or concomitantly with the last ketoconazole dose of 3 days treatment with ketoconazole 100 mg twice daily. Co-administration of ketoconazole resulted in 8-fold the AUC of budesonide, compared to budesonide alone.
In an open, randomized, cross-over study 8 healthy subjects were given Entocort EC 3 mg as a single dose, either alone or concomitantly with the last ketoconazole dose of 4 days treatment with ketoconazole 200 mg once daily. Co-administration of ketoconazole resulted in 6.5-fold the AUC of budesonide, compared to budesonide alone.
Grapefruit Juice
In an open, randomized, cross-over study, 8 healthy subjects were given Entocort EC 3 mg, either alone, or concomitantly with 600 mL concentrated grapefruit juice (which inhibits CYP3A4 activity predominantly in the intestinal mucosa), on the last of 4 daily administrations. Concomitant administration of grapefruit juice resulted in doubling the bioavailability of budesonide compared to budesonide alone.
Proton Pump Inhibitors
The pharmacokinetics of TARPEYO have not been evaluated in combination with proton pump inhibitors (PPIs). Since the disintegration of TARPEYO is pH dependent, the release properties and uptake of budesonide may be altered when TARPEYO is taken after treatment with PPIs. In a study assessing intragastric and intraduodenal pH in healthy volunteers after repeated dosing with the PPI omeprazole 40 mg once daily, intragastric and intraduodenal pH did not exceed that required for disintegration of TARPEYO. Beyond the duodenum, PPIs such as omeprazole are unlikely to affect pH.
Oral Contraceptives (CYP3A4 Substrates)
In a parallel study, the pharmacokinetics of budesonide were not significantly different between healthy female subjects who received oral contraceptives containing desogestrel 0.15 mg and ethinyl estradiol 30 μg and healthy female subjects who did not receive oral contraceptives. Budesonide 4.5 mg once daily for one week did not affect the plasma concentrations of ethinyl estradiol, a CYP3A4 substrate. The effect of budesonide 16 mg once daily on the plasma concentrations of desogestrel and ethinyl estradiol was not studied.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity studies with budesonide were conducted in rats and mice. In a two-year study in Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of gliomas in male rats at an oral dose of 50 mcg/kg (approximately 0.03 times the maximum recommended human dose (MRHD) on a body surface area basis). In addition, there were increased incidences of primary hepatocellular tumors in male rats at 25 mcg/kg (approximately 0.015 times the MRHD on a body surface area basis) and above. No tumorigenicity was seen in female rats at oral doses up to 50 mcg/kg (approximately 0.03 times the MRHD on a body surface area basis). In an additional two-year study in male Sprague-Dawley rats, budesonide caused no gliomas at an oral dose of 50 mcg/kg (approximately 0.03 times the MRHD on a body surface area basis). However, it caused a statistically significant increase in the incidence of hepatocellular tumors at an oral dose of 50 mcg/kg (approximately 0.03 times the MRHD of a body surface area basis). The concurrent reference corticosteroids (prednisolone and triamcinolone acetonide) showed similar findings. In a 91-week study in mice, budesonide caused no treatment-related carcinogenicity at oral doses up to 200 mcg/kg (approximately 0.06 times the MRHD on a body surface area basis).
Budesonide was not genotoxic in the Ames test, the mouse lymphoma cell forward gene mutation (TK+/-) test, the human lymphocyte chromosome aberration test, the Drosophila melanogaster sex-linked recessive lethal test, the rat hepatocyte UDS test and the mouse micronucleus test.
In rats, budesonide had no effect on fertility at subcutaneous doses up to 80 mcg/kg (approximately 0.05 times the MRHD on a body surface area basis). However, it caused a decrease in prenatal viability and viability in pups at birth and during lactation, along with a decrease in maternal food consumption and body weight gain, at subcutaneous doses of 20 mcg/kg (approximately 0.012 times the MRHD on a body surface area basis) and above. No such effects were noted at 5 mcg/kg (approximately 0.003 times the MRHD on a body surface area basis).
-
14 CLINICAL STUDIES
14.1 Treatment of IgAN
TARPEYO was shown to reduce the loss of kidney function in adults with primary IgAN at risk of disease progression in the NefIgArd trial. While the effect on kidney function that was seen during the 9-month treatment period persisted following completion of treatment, TARPEYO did not change the long-term rate of decline in kidney function.
NefIgArd Study: A Phase 3, Double-Blind Placebo-Controlled, Randomized Trial in Adults with Primary IgAN
The effect of TARPEYO on proteinuria and kidney function (estimated glomerular filtration rate, eGFR) was assessed in a randomized, double-blind, phase 3, 2-part, multicenter study (NefIgArd, NCT: 03643965) in adults with biopsy-proven IgAN, eGFR ≥35 mL/min/1.73 m2, and proteinuria (defined as either ≥1 g/day or urine protein to creatinine ratio (UPCR) ≥0.8 g/g) who were on a stable dose of maximally-tolerated RAS inhibitor therapy. Patients with other glomerulopathies, nephrotic syndrome, or those who had been treated with systemic immunosuppressive medications were excluded. Patients were randomized 1:1 to either TARPEYO 16 mg once daily or placebo and treated for nine months followed by a 2-week taper of either TARPEYO 8 mg once daily or placebo. Patients were then followed off-treatment for 15 months. The primary endpoint for Part A of the study (interim analysis) was the ratio of UPCR (based on 24-hour urine collections) at 9 months compared to baseline based on the first 199 randomized patients who completed the Month 9 visit. The primary endpoint for Part B of the study (final analysis) was a time-weighted average of the log ratio of eGFR at each time point over 2 years relative to baseline.
Of the 364 randomized patients evaluated for efficacy, 66% were male, 76% were Caucasian, 23% were Asian, and 20% were from North America. The median age was 43 years (range 20 to 73 years). At baseline, the mean eGFR was approximately 58 mL/min/1.73 m2, with 60% of patients having an eGFR <60 mL/min/1.73 m2. The mean baseline UPCR was 1.5 g/g and 21% of patients had proteinuria >3.5 g/24 hours. Approximately 70% of patients had a history of hypertension and 7% had a history of type 2 diabetes mellitus. At baseline, 98% were treated with an angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB) and <1% of patients were on a sodium-glucose cotransporter 2 (SGLT2) inhibitor. At study entry, the median systolic/diastolic blood pressure was 125/79 mmHg.
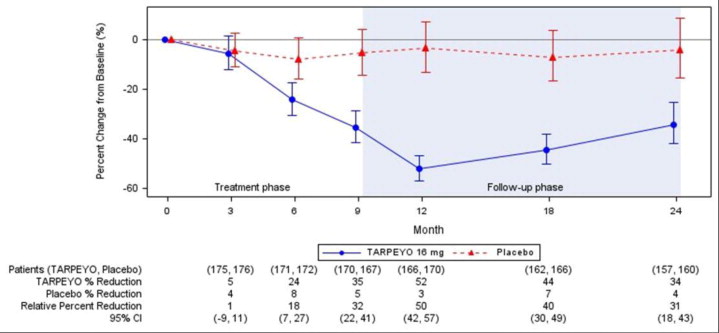
The trial met the prespecified Part A primary endpoint based on an interim analysis of 199 randomized patients who had completed the Month 9 visit. The interim analysis showed a 31% reduction in UPCR in patients treated with TARPEYO 16 mg once daily compared to placebo (95% CI: 16% to 42% reduction; p=0.0001). In the final analysis of 364 patients, the percentage change in UPCR observed at 9 months was consistent with the results in the subset of 199 patients included in the interim analysis. The final analysis of the percentage change in UPCR during the treatment and follow-up phase is shown in Figure 1.
Figure 1: LS Mean (95% CI) Percentage Change from Baseline in UPCR (g/g) in NefIgArd Study (Full Analysis Set)
Estimated mean percentage change from baseline in UPCR with 95% confidence intervals estimated from a mixed model repeated measures analysis of log-transformed post-baseline to baseline ratios at 3, 6, 9, 12, 18, and 24 months. Analysis included all UPCR data regardless of use of prohibited medication at any point during the study.
Values reported under the figure are converted to percent reduction from baseline. Relative percent reductions comparing TARPEYO and placebo are estimated from the regression model.
Abbreviations: UPCR, urine protein to creatinine ratio; CI, confidence interval; LS, least squares.
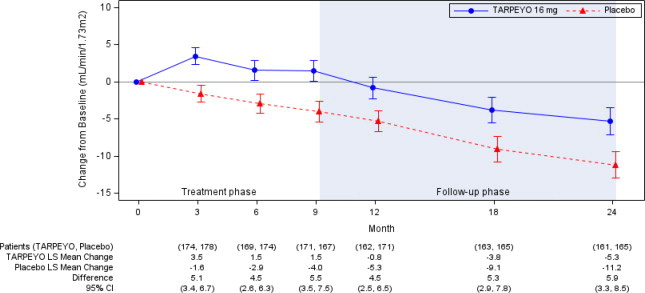
In the final analysis of 364 patients, the trial met the prespecified Part B primary endpoint (p<0.0001). The mean change from baseline in eGFR and respective 95% CI for each arm at each scheduled visit during the treatment and follow-up phase is shown in Figure 2. The favorable effect of TARPEYO on eGFR was seen by Month 3 (the earliest assessment) and did not appear to increase in magnitude over two years. At Year 2, there was a 5.9 mL/min/1.73 m2 difference in the mean change from baseline in eGFR between TARPEYO and placebo (95% CI: 3.3 to 8.5 mL/min/1.73 m2; p<0.0001).
Figure 2: LS Mean (95% CI) Change from Baseline in eGFR (mL/min/1.73 m2) in NefIgArd Study (Full Analysis Set)
Estimated least squares mean change from baseline in eGFR (mL/min/1.73 m2) with 95% confidence intervals estimated from a mixed model repeated measures analysis of post-baseline to baseline differences at 3, 6, 9, 12, 18, and 24 months. Analysis was based on untransformed data and includes all eGFR data regardless of use of prohibited medication at any point during the study. A total of 15 patients in the TARPEYO arm and 20 patients in the placebo arm received rescue medication during the 2-year study.
Abbreviations: eGFR, estimated glomerular filtration rate; CI, confidence interval; LS least squares
The treatment effect based on the change from baseline in eGFR at 2 years was consistent across key subgroups, including key demographic (such as age, sex, race) and baseline disease (such as baseline proteinuria) characteristics.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TARPEYO (budesonide) delayed release capsules 4 mg, are white opaque- coated capsules marked with “CAL10 4 MG” in black ink on the body of the capsule. They are supplied as follows:
NDC 81749-004-01: Bottles of 120 capsules. Child-resistant cap.
Store at 20-25°C (68 - 77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature].
Keep container tightly closed. Protect from moisture.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advise patients that TARPEYO may cause hypercorticism and adrenal axis suppression and to follow a taper schedule, as instructed by their healthcare provider if discontinuing therapy [see Warnings and Precautions (5.1)].
TARPEYO causes immunosuppression. Advise patients to avoid exposure to people with chicken pox or measles and, if exposed, to consult their healthcare provider immediately. There is an increased risk of developing a variety of infections, including worsening of existing tuberculosis, fungal, bacterial, viral or parasitic infections, or ocular herpes simplex, and to contact their healthcare provider if they develop any symptoms of infection [see Warnings and Precautions (5.3)]. Provide advice regarding vaccination schedules for immunocompromised patients.
Advise patients that TARPEYO delayed release capsules should be swallowed whole and not chewed, crushed or broken and to take TARPEYO in the morning, at least 1 hour before a meal [See Dosage and Administration (2)].
Advise patients to avoid the consumption of grapefruit juice for the duration of their TARPEYO therapy [See Drug Interactions (7.1)].
TARPEYO is a registered trademark of Calliditas Therapeutics AB, or its affiliates.
© 2024 Calliditas Therapeutics AB (publ)
Manufactured for and distributed by:
Calliditas Therapeutics ABStockholm, Sweden
Patent: http://www.calliditas.com/patents
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 12/2023
Patient Information
TARPEYO (tar-PAY-oh)
(budesonide)
delayed release capsulesWhat is TARPEYO?
TARPEYO is a prescription medicine used to reduce the loss of kidney function in adults with a kidney disease called primary immunoglobulin A nephropathy (IgAN), who are at risk for their disease getting worse.
It is not known if TARPEYO is safe and effective in children.Do not take TARPEYO if you are allergic to budesonide or any of the ingredients in TARPEYO. See the end of this leaflet for a complete list of ingredients in TARPEYO. Before taking TARPEYO, tell your healthcare provider about all of your medical conditions, including if you:
- have liver problems.
- plan to have surgery.
- have chickenpox or measles or have recently been near anyone with chickenpox or measles.
- have an infection.
- have high blood sugar levels (prediabetes or diabetes).
- have glaucoma or cataracts.
- have a family history of diabetes or glaucoma.
- have or have had tuberculosis.
- have high blood pressure (hypertension).
- have decreased bone mineral density (osteoporosis).
- have stomach ulcers.
- are pregnant or plan to become pregnant. TARPEYO may harm your unborn baby. Talk to your healthcare provider about the possible risk to your unborn baby if you take TARPEYO when you are pregnant.
- are breastfeeding or plan to breastfeed. It is not known if TARPEYO passes into your breast milk or if it will affect your baby. Talk to your healthcare provider about the best way to feed your baby during treatment with TARPEYO.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. TARPEYO and other medicines may affect each other causing side effects. How should I take TARPEYO?
- Take TARPEYO exactly as your healthcare provider tells you.
- Your healthcare provider will decide how long you should take TARPEYO. Do not stop taking TARPEYO without first talking with your healthcare provider.
- Take your prescribed dose of TARPEYO 1 time each day in the morning, at least 1 hour before a meal.
- Swallow TARPEYO capsules whole. Do not open, chew, crush, or break TARPEYO capsules before swallowing.
- If you miss a dose of TARPEYO, take your prescribed dose at your next scheduled time. Do not take two doses of TARPEYO at the same time.
- If you take too much TARPEYO, call your healthcare provider right away or go to the nearest hospital emergency room.
What should I avoid while taking TARPEYO?
Do not eat grapefruit or drink grapefruit juice during your treatment with TARPEYO. Eating grapefruit or drinking grapefruit juice can increase the level of TARPEYO in your blood.What are the possible side effects of TARPEYO?
TARPEYO may cause serious side effects, including:
- Effects of having too much corticosteroid medicine in your blood (hypercorticism). Long-time use of TARPEYO can cause you to have signs and symptoms of too much cortisol, a stress hormone in your blood. Tell your healthcare provider if you have any of the following signs and symptoms of hypercorticism:
- acne
- bruise easily
- rounding of your face (moon face)
- ankle swelling
- thicker or more hair on your body and face
- a fatty pad or hump between your shoulders (buffalo hump)
- pink or purple stretch marks on the skin of your abdomen, thighs, breasts, or arms
-
Adrenal suppression. When TARPEYO is taken for a long period of time (chronic use), adrenal suppression can happen. This is a condition in which the adrenal glands do not make enough steroid hormones. Symptoms of adrenal suppression include:
- tiredness
- weakness
- nausea and vomiting
- low blood pressure
Tell your healthcare provider if you are under stress or have any symptoms of adrenal suppression during treatment with TARPEYO.
- Risk of immunosuppression. TARPEYO weakens your immune system. Taking medicines that weaken your immune system makes you more likely to get infections. Avoid contact with people who have contagious diseases, such as chickenpox or measles, during treatment with TARPEYO. Tell your healthcare provider right away if you come in contact with anyone who has chickenpox or measles. Consult with your healthcare provider regarding appropriate vaccination scheduling.
- Tell your healthcare provider if you develop any symptoms of infection during treatment with TARPEYO, including:
- fever
- feeling tired
- chills
- aches
- pain
- nausea and vomiting
The most common side effects of TARPEYO include: - swelling of the lower legs, ankles, and feet
- high blood pressure
- muscle spasms
- acne
- headache
- upper respiratory tract infection
- swelling of the face
- weight increase
- indigestion
- irritation or inflammation of the skin
- joint pain
- increased white blood cell count
These are not all the possible side effects of TARPEYO.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store TARPEYO?
- Store TARPEYO at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep TARPEYO in a tightly closed container.
- Protect from moisture.
Keep TARPEYO and all medicines out of the reach of children. General information about the safe and effective use of TARPEYO.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use TARPEYO for a condition for which it was not prescribed. Do not give TARPEYO to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about TARPEYO that is written for health professionals.What are the ingredients in TARPEYO?
Active ingredient: budesonide
Inactive ingredients: sugar spheres (sucrose and starch), hypromellose, polyethylene glycol, citric acid monohydrate, ethyl cellulose, medium chain triglycerides and oleic acid.
The capsules contain: hypromellose and titanium oxide (E171).
The printing ink on the capsules contain: shellac, propylene glycol and black iron oxide (E172).
The enteric coating on the capsules contain: methacrylic acid and methacrylate copolymer, talc and dibutyl sebacate.Manufactured for and distributed by: Calliditas Therapeutics AB, Stockholm, Sweden
TARPEYO is a trademark of Calliditas Therapeutics AB, or its affiliates.
© 2021 Calliditas Therapeutics AB (publ)
Patent: http://www.calliditas.com/patents
For more information, go to www.TARPEYOTouchpoints.com or call 1-933-444-8277. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TARPEYO
budesonide capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81749-004 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength budesonide (UNII: Q3OKS62Q6X) (budesonide - UNII:Q3OKS62Q6X) budesonide 4 mg Inactive Ingredients Ingredient Name Strength Sucrose (UNII: C151H8M554) Starch, Corn (UNII: O8232NY3SJ) Hypromellose 2910 (5 MPA.S) (UNII: R75537T0T4) Hypromellose, unspecified (UNII: 3NXW29V3WO) hypromellose 2910 (3 MPA.S) (UNII: 0VUT3PMY82) Polyethylene glycol 400 (UNII: B697894SGQ) Citric acid monohydrate (UNII: 2968PHW8QP) Ethylcellulose (10 MPA.S) (UNII: 3DYK7UYZ62) Medium-chain triglycerides (UNII: C9H2L21V7U) Oleic acid (UNII: 2UMI9U37CP) Titanium dioxide (UNII: 15FIX9V2JP) Shellac (UNII: 46N107B71O) Propylene glycol (UNII: 6DC9Q167V3) Ferrosoferric oxide (UNII: XM0M87F357) Methacrylic Acid - Methyl Methacrylate Copolymer (1:2) (UNII: 5KY68S2577) Methacrylic Acid - Methyl Methacrylate Copolymer (1:1) (UNII: 74G4R6TH13) Talc (UNII: 7SEV7J4R1U) Dibutyl sebacate (UNII: 4W5IH7FLNY) Product Characteristics Color white (white) Score no score Shape CAPSULE (CAPSULE) Size 19mm Flavor Imprint Code CAL10;4MG Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81749-004-01 120 in 1 BOTTLE; Type 0: Not a Combination Product 12/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215935 12/15/2021 Labeler - Calliditas Therapeutics AB (351157735) Establishment Name Address ID/FEI Business Operations Micro-Macinazione S.A. 480918515 PARTICLE SIZE REDUCTION(81749-004) Establishment Name Address ID/FEI Business Operations Minakem Dunkerque Production S.A.S 277412599 API MANUFACTURE(81749-004) Establishment Name Address ID/FEI Business Operations Patheon Pharmaceuticals Inc. 005286822 MANUFACTURE(81749-004) Establishment Name Address ID/FEI Business Operations Sicor Società Italiana Corticosteroidi S.r.l 338950678 API MANUFACTURE(81749-004)