Label: GMS ELECTROLYTE SOLUTION COMPOUND SODIUM LACTATE HARTMANNS- sodium chloride, potassium chloride, sodium lactate and calcium chloride solution
- NDC Code(s): 70346-100-05
- Packager: GM Scientific, LLC
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 22, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STERILE NONPYROGENIC SOLUTION For Animal Use Only
-
Description
GMS Electrolyte Solution Compound Sodium Lactate (Hartmanns Solution) is a sterile, non-pyrogenic solution intended for fluid and electrolyte replenishment in single dose containers. May be administered intravenously, subcutaneously or intraperitoneally (except in horses) using aseptic technique. It contains no antimicrobial agents. Discard any unused portion. Composition, osmolarity, pH and ionic concentration are shown in Table 1.
Table 1
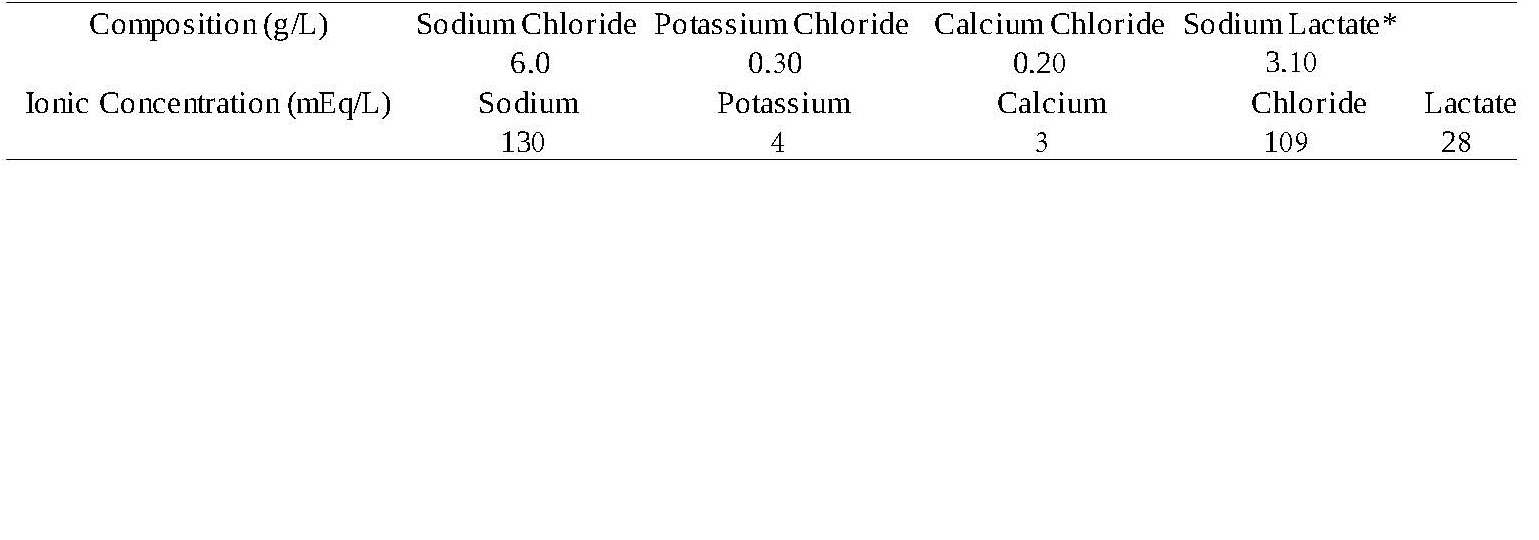
*Sodium Lactate USP (S)-enantiomer
Osmolarity (mOsmol/L) (calc): 280mOsmol per litre
pH: 6.5 (limit 6.0 7.0)
The container is free of PVC and phthalates. It is fabricated from 5 layer polyolefin based co-extrusion material. The container meets the requirements of USP (Class VI).
- Clinical Pharmacology
- Indications
- Contraindications
-
Warnings
The introduction of additives to any solution, regardless of type of container, requires special attention to ensure that no incompatibilities result. While some incompatibilities are readily absorbed, one must be aware that subtle physical, chemical and pharmacological incompatibilities can occur. The medical literature, the package insert and other available sources of information should be reviewed for thorough understanding of possible incompatibilities.
GMS Electrolyte Solution Compound Sodium Lactate (Hartmann’s Solution) should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency, and in clinical states in which there exists edema and sodium retention.
GMS Electrolyte Solution Compound Sodium Lactate (Hartmann’s Solution) should be used with great care, if at all, in patients with hyperkalemia, severe renal failure, and in conditions in which potassium retention is present.
GMS Electrolyte Solution Compound Sodium Lactate (Hartmann’s Solution) should be used with great care in patients with metabolic or respiratory alkalosis. The administration of lactate ions should be done with great care in those conditions in which there is an increased level or an impaired utilization of these ions, such as severe hepatic insufficiency.
GMS Electrolyte Solution Compound Sodium Lactate (Hartmann’s Solution) should not be administered simultaneously with blood through the same administration set because of likelihood of coagulation.
The intravenous administration of GMS Electrolyte Solution Compound Sodium Lactate (Hartmann’s Solution) can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, over-hydration, congested states, or pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentrations of the injections. The risk of solute overloading causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of the injections.
In patients with diminished renal function, administration of GMS Electrolyte Solution Compound Sodium Lactate (Hartmann’s Solution) may result in sodium or potassium retention.
Do not administer to horses by intraperitoneal injection. Do not administer to animals with inadequate renal function. Not for use in lactic acidosis.
-
Adverse Reactions
Adverse reactions may occur due to the solution or the technique of administration including fever response, infection at the site of injection or allergic reactions. Prolonged intravenous infusion of this type of product may cause venous thrombosis or phlebitis extending from the site of injection, extravasation, and hypervolemia.
If an adverse reaction does occur, discontinue the infusion and evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
-
Precautions
This is a single dose unit. It contains no preservatives. Use entire contents when first opened.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid base balance during prolonged therapy or whenever the condition of the patient warrants such evaluation.
GMS Electrolyte Solution Compound Sodium Lactate (Hartmann’s Solution) should be used with caution. Excess administration may result in metabolic alkalosis.
Do not administer unless solution is clear and both seal and container are intact.
Solution must be warmed to body temperature prior to administration and administered at a slow rate. Use solution promptly following initial entry.
Reactions which may occur because of the solution or the technique of administration, include febrile response, infection at the site of injection, extravasation, and hypervolemia.
If an adverse reaction does occur, discontinue the infusion and evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
-
Dosage and Administration
To be used as directed by a licensed veterinarian. The dosage of the GMS Electrolyte Solution Compound Sodium Lactate (Hartmann’s Solution) is dependent upon the age, weight and clinical conditions of the patient as well as laboratory determinations. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
For use in one patient on one occasion only. Discard any unused portion. Care should be taken with administration technique to avoid administration site reactions and infection.
Additives may be incompatible. Complete information is not available. Those additives known to be incompatible should not be used. Consult with Pharmacist, if available. If, in the informed judgement of the doctor, it is deemed advisable to introduce additives, use aseptic technique. Mix thoroughly when additives have been introduced. Do not store solutions containing additives.
- Over-dosage
-
Packs Supplied
GMS Electrolyte Solution Compound Sodium Lactate (Hartmann’s Solution) in plastic container is available as follows:
Size (mL)
Item Code
NDC
5000
EQLRS-5
70346-100-05
Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (30°C/86°F). Protect from freezing.
-
Directions for use of plastic container
To Open
Tear overwrap at slit and remove solution container. Some opacity of the plastic due to moisture absorption during the sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually. Check for minute leaks by squeezing solution container firmly. If leaks are found, discard solution as sterility may be impaired. If supplemental medication is desired, follow directions below:
Preparation for Administration
- Suspend container from eyelet support.
- Remove plastic protector from inlet/outlet port at bottom of container.
- Attach administration set.
To Add Medication
WARNING: Additives may be incompatible.
To add medication before solution administration
- Prepare medication site.
- Using syringe with 0.63mm to 0.80mm needle, puncture medication port and inject.
- Mix solution and medication thoroughly. For high density medication such as potassium chloride, squeeze ports while ports are upright and mix thoroughly.
To add medication during solution administration
- Close the clamp on the administration set.
- Prepare medication site.
- Using syringe with 0.63mm to 0.80mm needle, puncture medication port and inject.
- Remove container from IV pole and/or turn to an upright position.
- Evacuate both ports by squeezing them while container is in the upright position.
- Mix solution and medication thoroughly.
- Return container to in use position and continue administration.
CAUTION: FEDERAL LAW RESTRICTS THIS DRUG TO USE BY OR ON THE ORDER OF A LICENSED VETERINARIAN.
Distributed by:
GM Scientific LLC.
736 N. Western Avenue, Lake Forest, Illinois 60045, USA
For customer service email:
leigh@gmscientific.com
Version: US_01
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GMS ELECTROLYTE SOLUTION COMPOUND SODIUM LACTATE HARTMANNS
sodium chloride, potassium chloride, sodium lactate and calcium chloride solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:70346-100 Route of Administration INTRAVENOUS, SUBCUTANEOUS, INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698, SODIUM CATION - UNII:LYR4M0NH37) SODIUM CHLORIDE 600 mg in 100 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152, CHLORIDE ION - UNII:Q32ZN48698) POTASSIUM CHLORIDE 30 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 20 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (LACTIC ACID - UNII:33X04XA5AT, SODIUM CATION - UNII:LYR4M0NH37) SODIUM LACTATE 310 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70346-100-05 5000 mL in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/22/2015 Labeler - GM Scientific, LLC (078713958) Registrant - GM Scientific, LLC (078713958)


