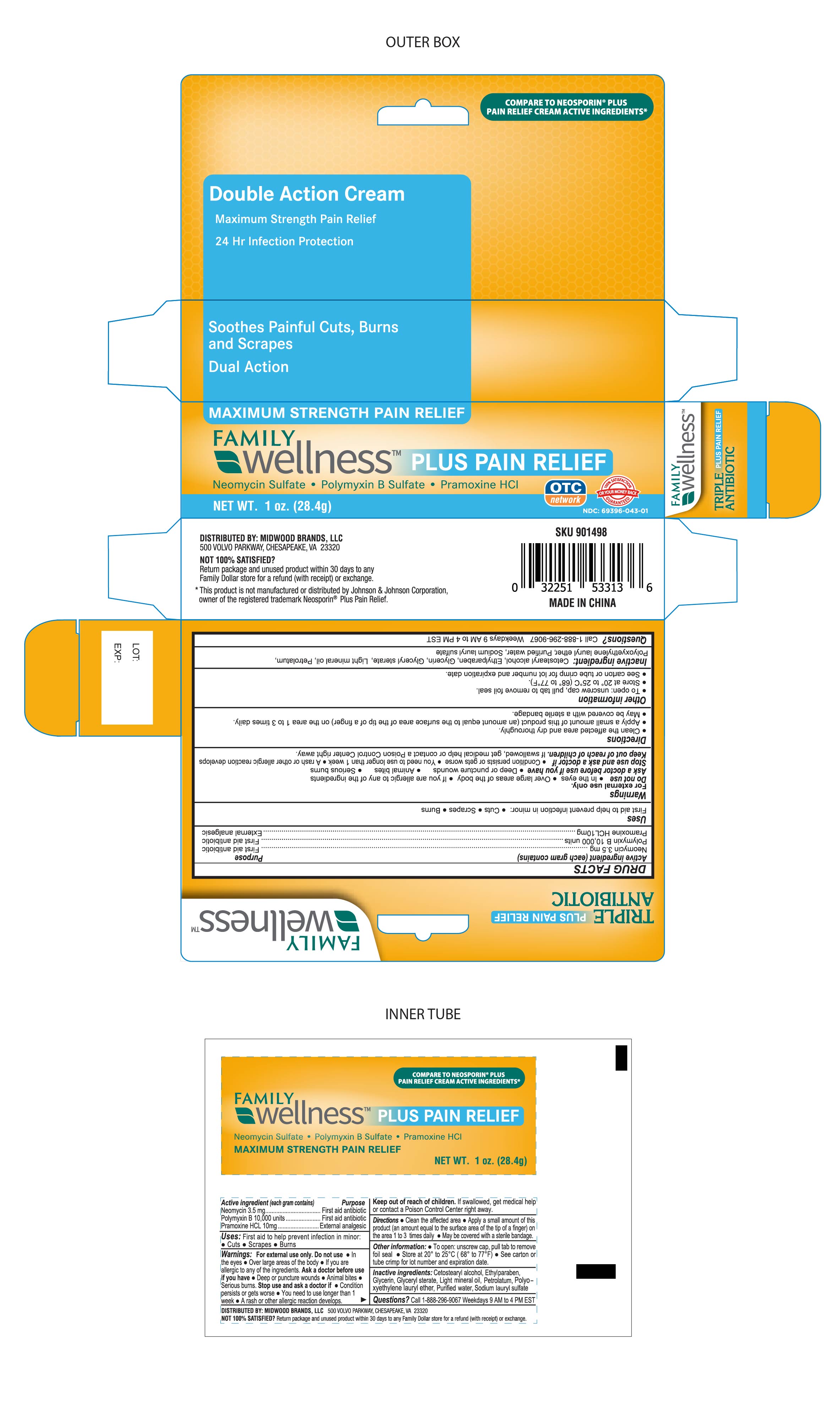
Label: FAMILY WELLNESS PLUS PAIN RELIEF- family wellness neomycin,polymyxinb,pramoxinehcl cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 69396-043-01 - Packager: Trifecta Pharmaceutical USA LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 5, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DRUG FACTS
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Active Ingredient
- Purpose
- Uses
- Warnings
- Ask a Doctor before Use
- Stop Use and ask a Doctor if:
- Keep out of Reach of Children
- Directions
- Other Information
- Inactive Ingredient:
-
Questions?
Call 1-888-296-9067
Weekdays 9AM - 4PM EST
DISTRIBUTED BY: MIDWOOD BRANDS, LLC.
500 Volvo Parkway, Chesapeake, VA. 23320
NOT SATISFIED?
Return package and unused product within 30 days to any Family Dollar store for a refund (with receipt) or exchange.
This product is not manufactured or distributed by Johnson & Johnson Corporation, owner of the registered trademark Neosporin® Plus Pain Relief.
MADE IN CHINA
- Packaging
-
INGREDIENTS AND APPEARANCE
FAMILY WELLNESS PLUS PAIN RELIEF
family wellness neomycin,polymyxinb,pramoxinehcl creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69396-043 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN SULFATE 3.5 mg in 1 g PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 10 mg in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 10000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength LAURYL ALCOHOL (UNII: 178A96NLP2) LIGHT MINERAL OIL (UNII: N6K5787QVP) SODIUM DODECYLBENZENESULFONATE (UNII: 554127163Y) PETROLATUM (UNII: 4T6H12BN9U) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) WATER (UNII: 059QF0KO0R) ETHYLPARABEN (UNII: 14255EXE39) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69396-043-01 1 in 1 BOX 09/22/2019 1 28.4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333B 08/30/2019 Labeler - Trifecta Pharmaceutical USA LLC (079424163)