Label: REPOVE R.E.P- glycerin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 73345-0030-1 - Packager: Binotec Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph not final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 24, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Eucalyptus Globulus Leaf Water
Laurus Nobilis Leaf Water
Butylene Glycol
Water
1,2-Hexanediol
Propanediol
Macadamia Ternifolia Seed Oil
Simmondsia Chinensis (Jojoba) Seed Oil
Propylene Glycol Dibenzoate
Heptyl Undecylenate
Niacinamide
Cyclodextrin
Sorbitol
Rosa Canina Fruit Oil
Adenosine
Polyglyceryl-10 Oleate
Glyceryl Distearate
Caprylic/Capric Triglyceride
Alcohol
Hydrogenated lecithin
Cholesterol
Ubiquinone
Thioctic Acid
Glutathione
Tocopherol
Ascorbic Acid
Copper Tripeptide-1
Palmitoyl Pentapeptide-4
Acetyl Hexapeptide-8
Palmitoyl Tripeptide-5
Alanine/Histidine/Lysine Polypeptide Copper HCl
Cetearyl Stearate
Hydrogenated Polydecene
Trideceth-10
Polyglyceryl-3 Diisostearate
Sorbitan Stearate
Glyceryl Stearate
Polyglyceryl-2 Stearate
Stearyl Alcohol
Carbomer
Sodium Chloride
Sodium Polyacryloyldimethyl Taurate
Ammonium Acryloyldimethyltaurate/Beheneth-25 Methacrylate Crosspolymer
lanthus Emblica Fruit Extract
Rubus Fruticosus (Blackberry) Fruit Extract
Euterpe Oleracea Fruit Extract
Rubus Idaeus (Raspberry) Fruit Extract
ium Angustifolium (Blueberry) Fruit Extract
Vaccinium Myrtillus Fruit Extract
Vaccinium Macrocarpon (Cranberry) Fruit Extract
Camellia Sinensis Leaf Extract
Solanum Tuberosum (Potato) Pulp Extract
Origanum Vulgare Leaf Extract
Mentha Viridis (Spearmint) Extract
Melissa Officinalis Extract
Pinus Koraiensis Seed Extract
Hovenia Dulcis Fruit Extract
Thuja Orientalis Seed Extract
Ginkgo Biloba Nut Extract
Myrciaria Dubia Seed Extract
Terminalia Ferdinandiana Fruit Extract
Adansonia Digitata Seed Extract
Coffea Arabica (Coffee) Seed Extract
Aleurites Moluccana Seed Extract
Macadamia Ternifolia Seed Extract
Madecassoside
Dimethyl Sulfone
Morinda Citrifolia Extract
Sodium Hyaluronate
Disodium EDTA
sh-Oligopeptide-1
sh-Polypeptide-1
sh-Polypeptide-3
Histidine
Arginine
Aspartic Acid
Threonine
Serine
Glutamic Acid
Proline
Glycine
Alanine
Valine
Methionine
Isoleucine
Leucine
Tyrosine
Phenylalanine
Lysine
Cysteine
Panax Ginseng Callus Culture Extract
Nelumbo Nucifera Callus Culture Extract
Solanum Lycopersicum (Tomato) Callus Culture Extract
Neofinetia Falcata Callus Culture Extract
Oryza Sativa (Rice) Callus Culture Extract
Ipomoea Hederacea Callus Culture Extract
Ethylhexylglycerin - PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
-
WARNINGS
If skin shows the following reactions while using 3G AB_Solution, reduce the amount of use drastically or discontinue use.
1. If itching continues for 7-10 days
2. If skin experiences a burning sensation or becomes reddish (dry skin type, particularly extremely dry skin)
3. If dead skin cells are generated drastically
* If you are receiving skin treatment due to skin diseases consult your doctor before use.
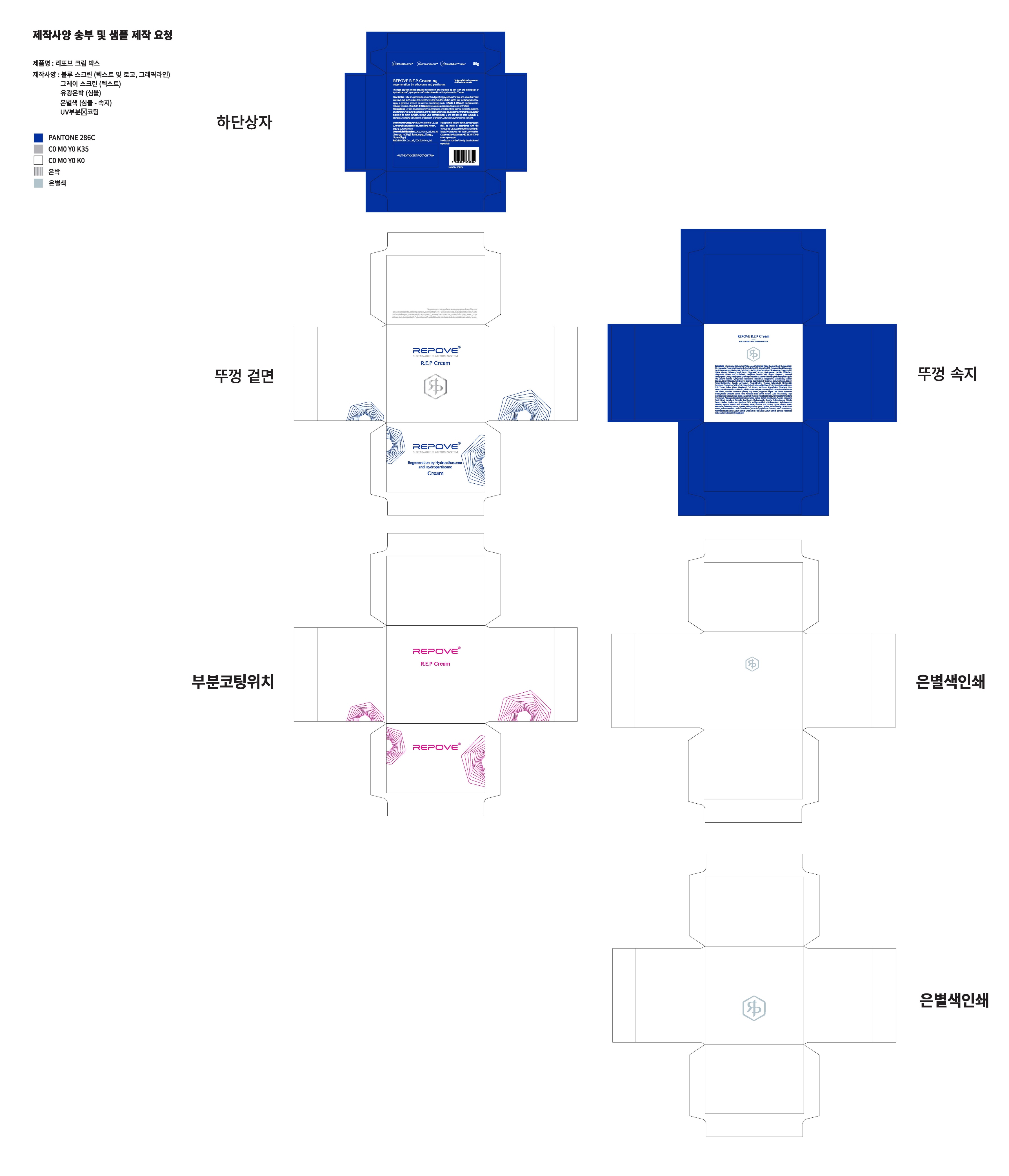
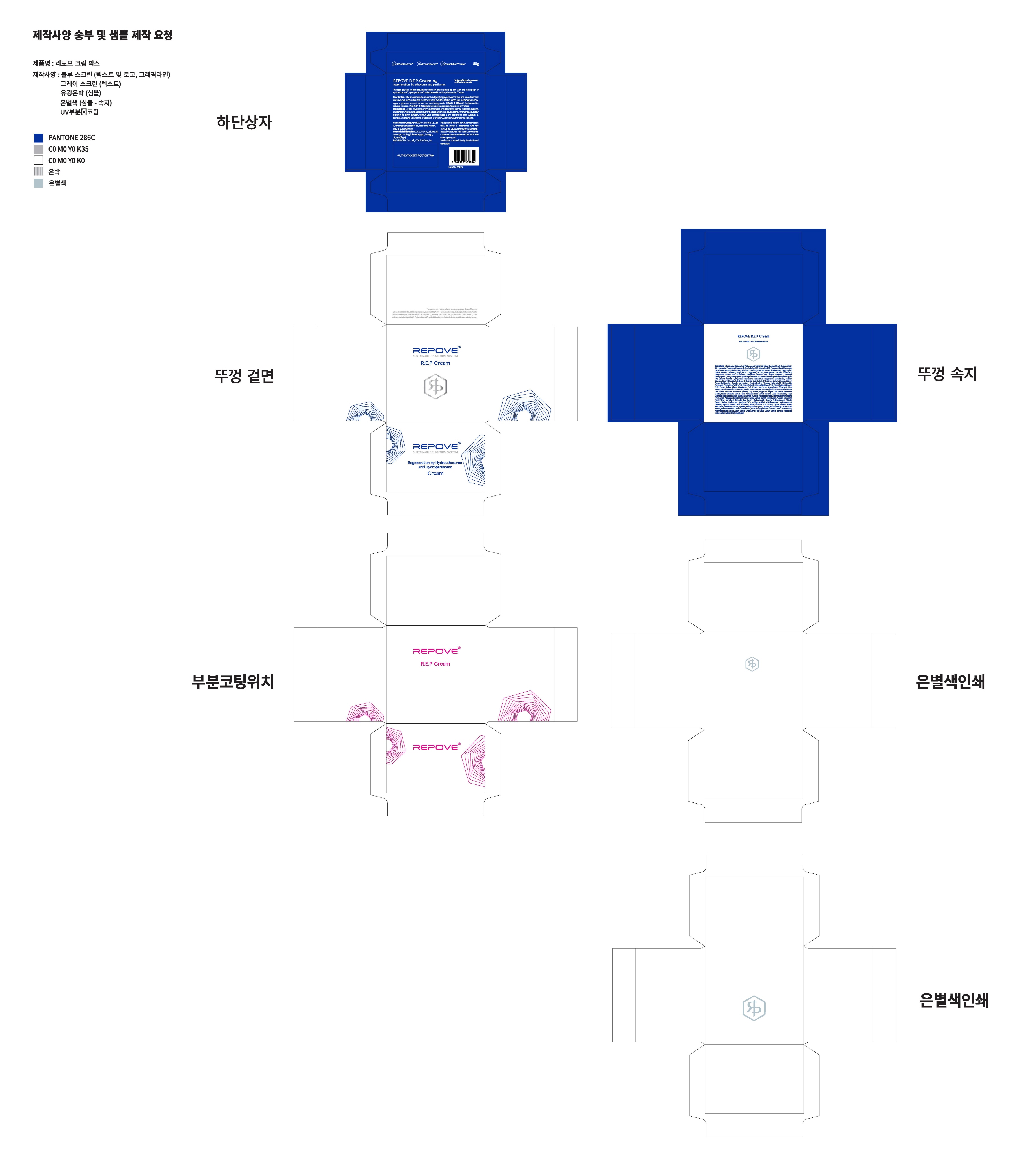
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
REPOVE R.E.P
glycerin creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73345-0030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 4 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ADENOSINE (UNII: K72T3FS567) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73345-0030-1 50 g in 1 JAR; Type 0: Not a Combination Product 08/01/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part347 08/01/2019 Labeler - Binotec Co., Ltd. (689840563) Registrant - Binotec Co., Ltd. (689840563) Establishment Name Address ID/FEI Business Operations reBom cosmetics co,.Ltd 688733595 pack(73345-0030) , manufacture(73345-0030) Establishment Name Address ID/FEI Business Operations Binotec Co., Ltd. 689840563 label(73345-0030)