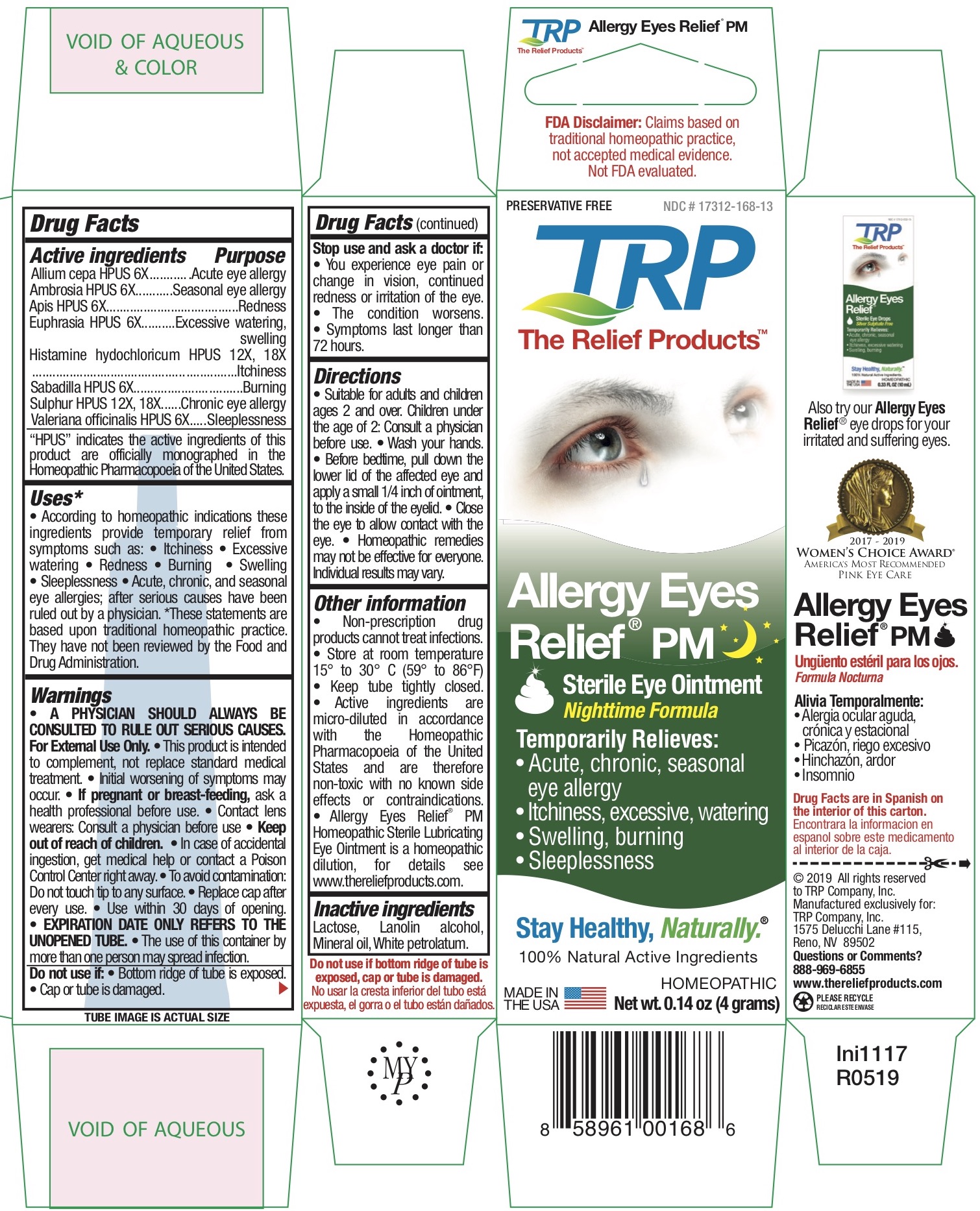
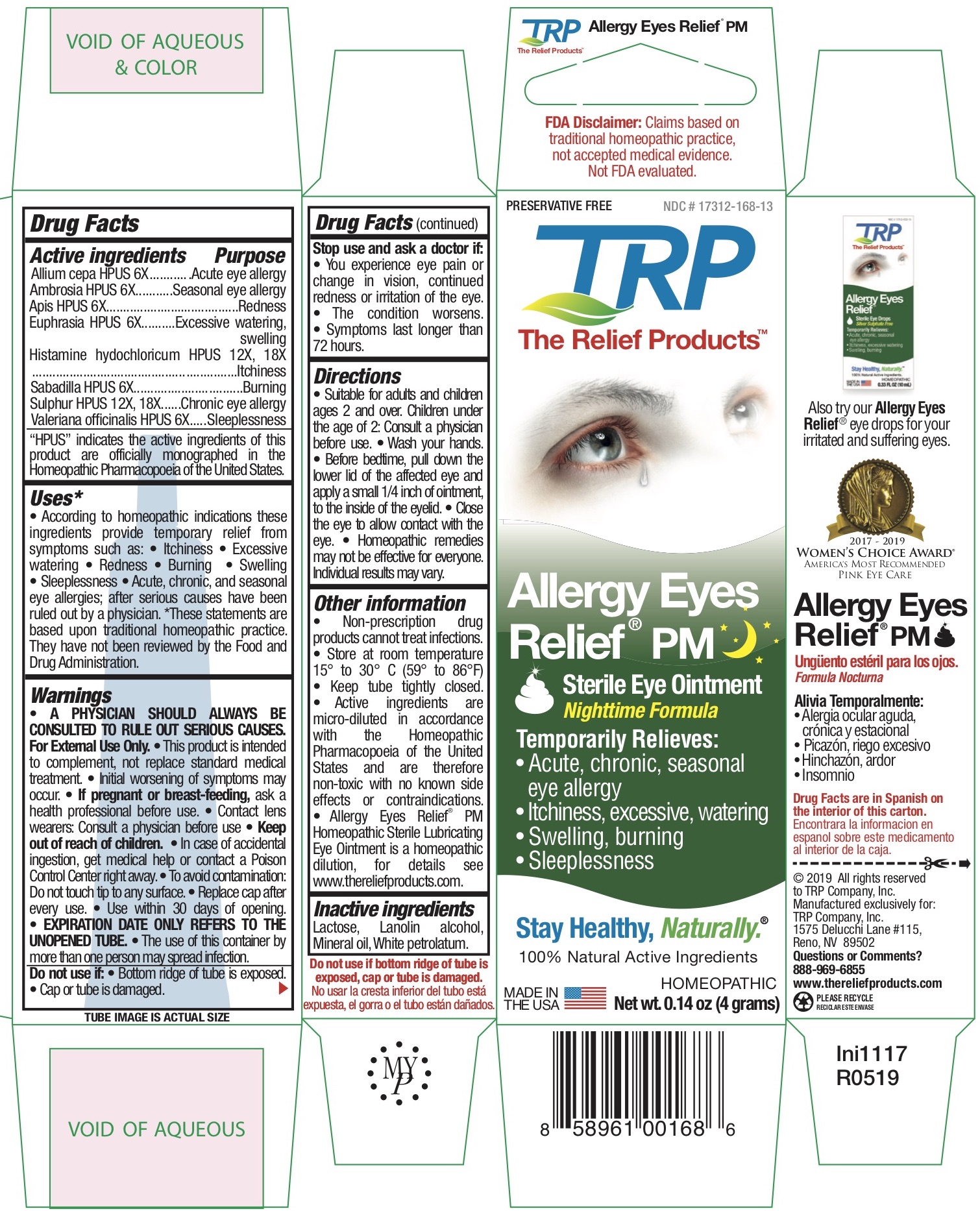
Label: ALLERGY EYES RELIEF NIGHTTIME FORMULA- allium cepa, ambrosia, apis, euphrasia, histamine hydochloricum, sabadilla, sulphur, valeriana officinalis ointment
- NDC Code(s): 17312-168-13
- Packager: TRP Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 10, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Allium cepa HPUS 6X........................................Acute eye allergy
Ambrosia HPUS 6X...........................................Seasonal eye allergy
Apis HPUS 6X...................................................Redness
Euphrasia HPUS 6X..........................................Excessive watering, swelling
Histamine hydochloricum HPUS 12X, 18X .......Itchiness
Sabadilla HPUS 6X............................................Burning
Sulphur HPUS 12X, 18X....................................Chronic eye allergy
Valeriana officinalis HPUS 6X.............................SleeplessnessThe letters HPUS indicate that the components of this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
- PURPOSE
-
INDICATIONS & USAGE
Uses*
According to homeopathic indications these ingredients provide temporary relief from symptoms such as: • Itchiness • Excessive watering • Redness • Burning • Swelling • Sleeplessness • Acute, chronic, and seasonal eye allergies; after serious causes have been ruled out by a physician.
*These statements are based upon traditional homeopathic practice. They have not been reviewed by the Food and Drug Administration. - WARNINGS
- PREGNANCY OR BREAST FEEDING
- WARNINGS
- Do not use:
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
DOSAGE & ADMINISTRATION
Directions:
• Suitable for adults and children ages 2 and over. Children under the age of 2: Consult a physician before use. • Wash your hands. • Squeeze out approx. 1/8” of ointment onto fingertip. • Gently apply the ointment directly to the eye. • Apply before bedtime. • Homeopathic remedies may not be effective for everyone. Individual results may vary.
-
SPL UNCLASSIFIED SECTION
Other information
• Store at room temperature 15° to 30° C (59° to 86°F) • Keep tube tightly closed. • Active ingredients are micro-diluted in accordance with the Homeopathic Pharmacopoeia of the United States and are therefore non-toxic with no known side effects or contraindications. • Allergy Eyes Relief TM Homeopathic Sterile Lubricating Eye Ointment is a homeopathic dilution, for details see www.thereliefproducts.com.
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALLERGY EYES RELIEF NIGHTTIME FORMULA
allium cepa, ambrosia, apis, euphrasia, histamine hydochloricum, sabadilla, sulphur, valeriana officinalis ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:17312-168 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HISTAMINE DIHYDROCHLORIDE (UNII: 3POA0Q644U) (HISTAMINE - UNII:820484N8I3) HISTAMINE DIHYDROCHLORIDE 12 [hp_X] in 4 g SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 12 [hp_X] in 4 g ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 6 [hp_X] in 4 g VALERIAN (UNII: JWF5YAW3QW) (VALERIAN - UNII:JWF5YAW3QW) VALERIAN 6 [hp_X] in 4 g SCHOENOCAULON OFFICINALE SEED (UNII: 6NAF1689IO) (SCHOENOCAULON OFFICINALE SEED - UNII:6NAF1689IO) SCHOENOCAULON OFFICINALE SEED 6 [hp_X] in 4 g AMBROSIA ARTEMISIIFOLIA (UNII: 9W34L2CQ9A) (AMBROSIA ARTEMISIIFOLIA - UNII:9W34L2CQ9A) AMBROSIA ARTEMISIIFOLIA 6 [hp_X] in 4 g EUPHRASIA STRICTA (UNII: C9642I91WL) (EUPHRASIA STRICTA - UNII:C9642I91WL) EUPHRASIA STRICTA 6 [hp_X] in 4 g APIS MELLIFERA (UNII: 7S82P3R43Z) (APIS MELLIFERA - UNII:7S82P3R43Z) APIS MELLIFERA 6 [hp_X] in 4 g Inactive Ingredients Ingredient Name Strength LACTOSE (UNII: J2B2A4N98G) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17312-168-13 1 in 1 PACKAGE 04/15/2019 1 4 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 04/15/2019 Labeler - TRP Company (105185719)