Label: ADELLINA TATTOO NUMBING- lidocaine cream
- NDC Code(s): 83596-001-01
- Packager: Aramode
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated November 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
- When using this product
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Storage and Handling:
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
ADELLINA TATTOO NUMBING
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83596-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 5 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) GLYCERIN (UNII: PDC6A3C0OX) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) MENTHA ARVENSIS LEAF (UNII: A4IWO4DDZ9) CHRYSANTHELLUM INDICUM TOP (UNII: STJ856D1Z0) PURSLANE (UNII: M6S840WXG5) POLYOXYL 40 HYDROGENATED CASTOR OIL (UNII: 7YC686GQ8F) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83596-001-01 1 in 1 PACKAGE 07/28/2023 1 50 g in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 07/28/2023 Labeler - Aramode (963192477) Registrant - Aramode (963192477) Establishment Name Address ID/FEI Business Operations Aramode 963192477 manufacture(83596-001)

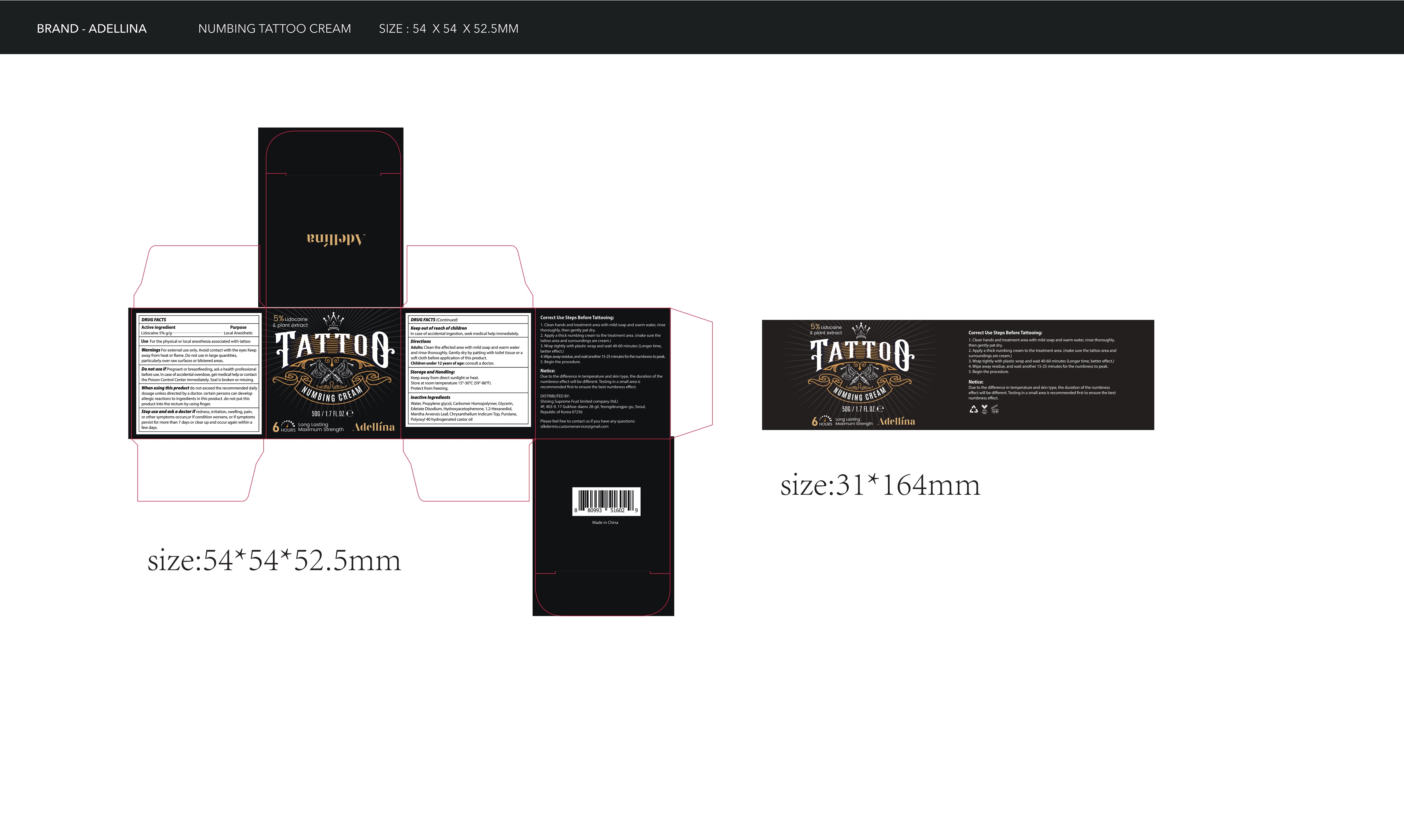
 50g NDC: 83596-001-01
50g NDC: 83596-001-01