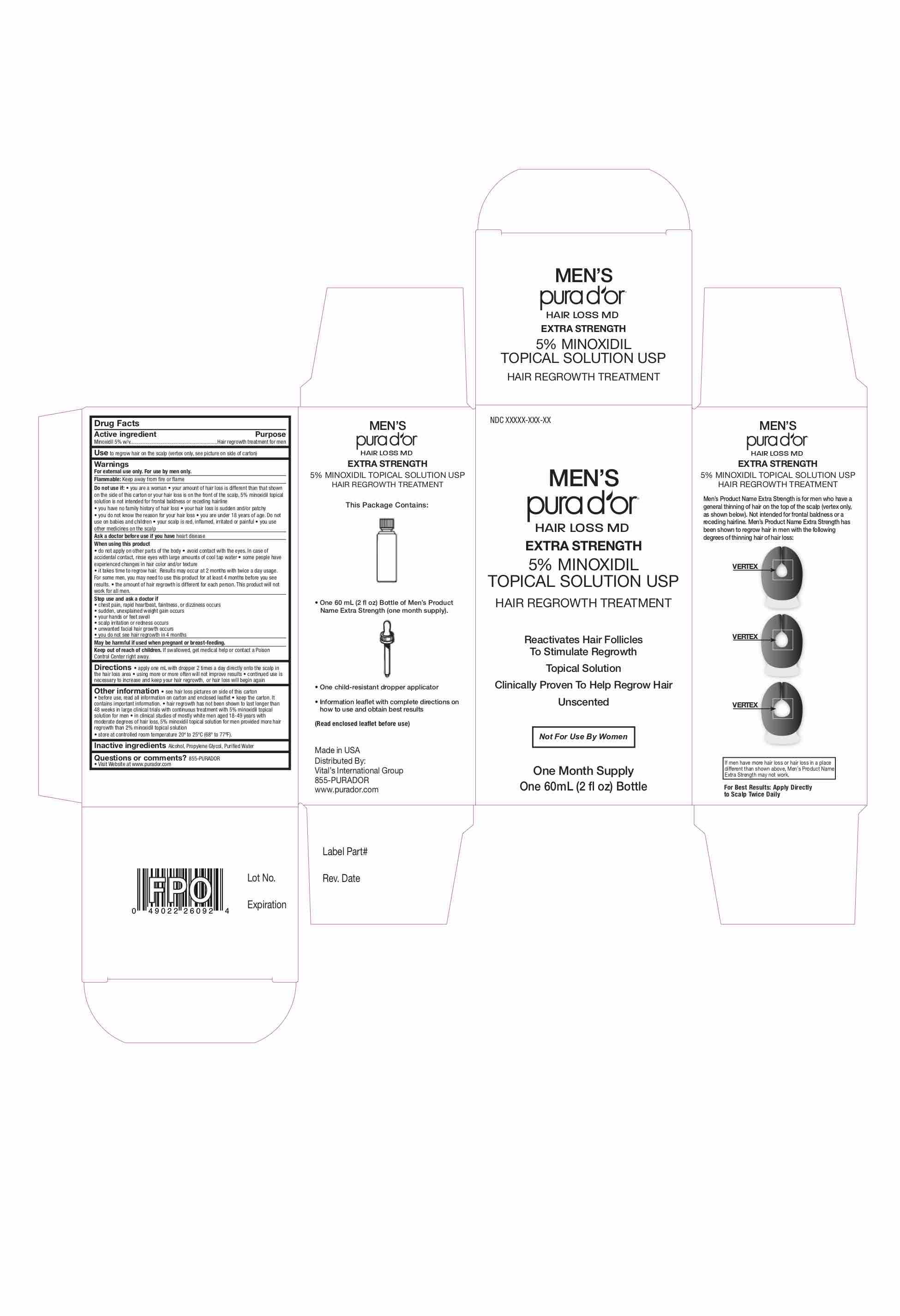
Label: MENS PURA DOR HAIR LOSS MD- minoxidil 5% solution
- NDC Code(s): 69019-001-01
- Packager: Vitals International Group
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
-
DO NOT USE
Do not use if: • you are a woman • your amount of hair loss is different than that shown
on the side of this carton or your hair loss is on the front of the scalp, 5% minoxidil topical
solution is not intended for frontal baldness or receding hairline
• you have no family history of hair loss • your hair loss is sudden and/or patchy
• you do not know the reason for your hair loss • you are under 18 years of age. Do not
use on babies and children • your scalp is red, inflamed, irritated or painful • you use
other medicines on the scalp
- ASK DOCTOR
-
WHEN USING
When using this product
• do not apply on other parts of the body • avoid contact with the eyes. In case of
accidental contact, rinse eyes with large amounts of cool tap water • some people have
experienced changes in hair color and/or texture
• it takes time to regrow hair. Results may occur at 2 months with twice a day usage.
For some men, you may need to use this product for at least 4 months before you see
results. • the amount of hair regrowth is different for each person. This product will not
work for all men.
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DIRECTIONS
-
OTHER SAFETY INFORMATION
Other information • see hair loss pictures on side of this carton
• before use, read all information on carton and enclosed leaflet • keep the carton. It
contains important information. • hair regrowth has not been shown to last longer than
48 weeks in large clinical trials with continuous treatment with 5% minoxidil topical
solution for men • in clinical studies of mostly white men aged 18-49 years with
moderate degrees of hair loss, 5% minoxidil topical solution for men provided more hair
regrowth than 2% minoxidil topical solution
• store at controlled room temperature 20º to 25ºC (68º to 77ºF).
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MENS PURA DOR HAIR LOSS MD
minoxidil 5% solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:69019-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ISOPROPYL ALCOHOL (UNII: ND2M416302) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:69019-001-01 1 in 1 BOX 09/27/2019 1 60 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076239 09/27/2019 Labeler - Vitals International Group (049656991)