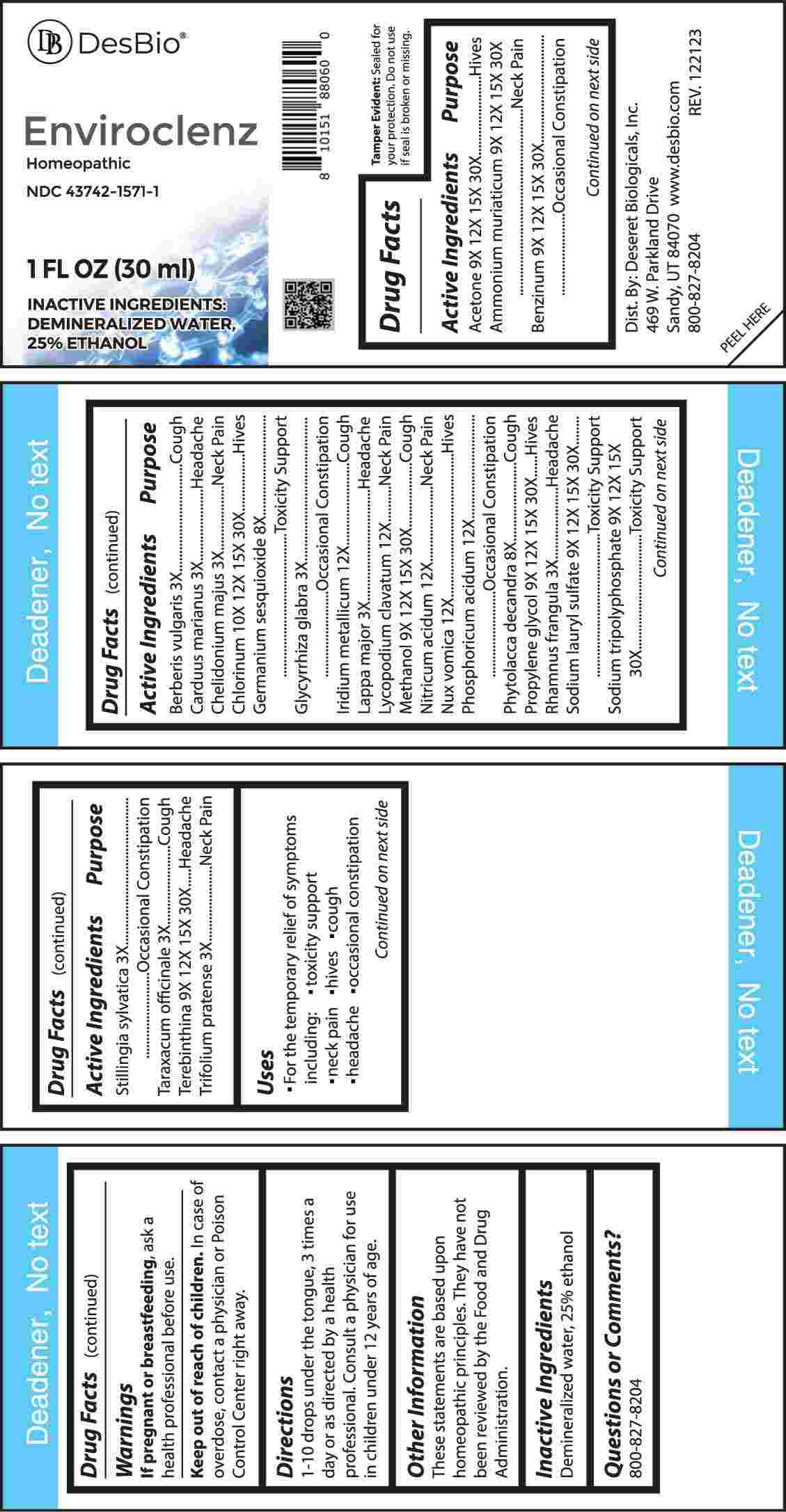
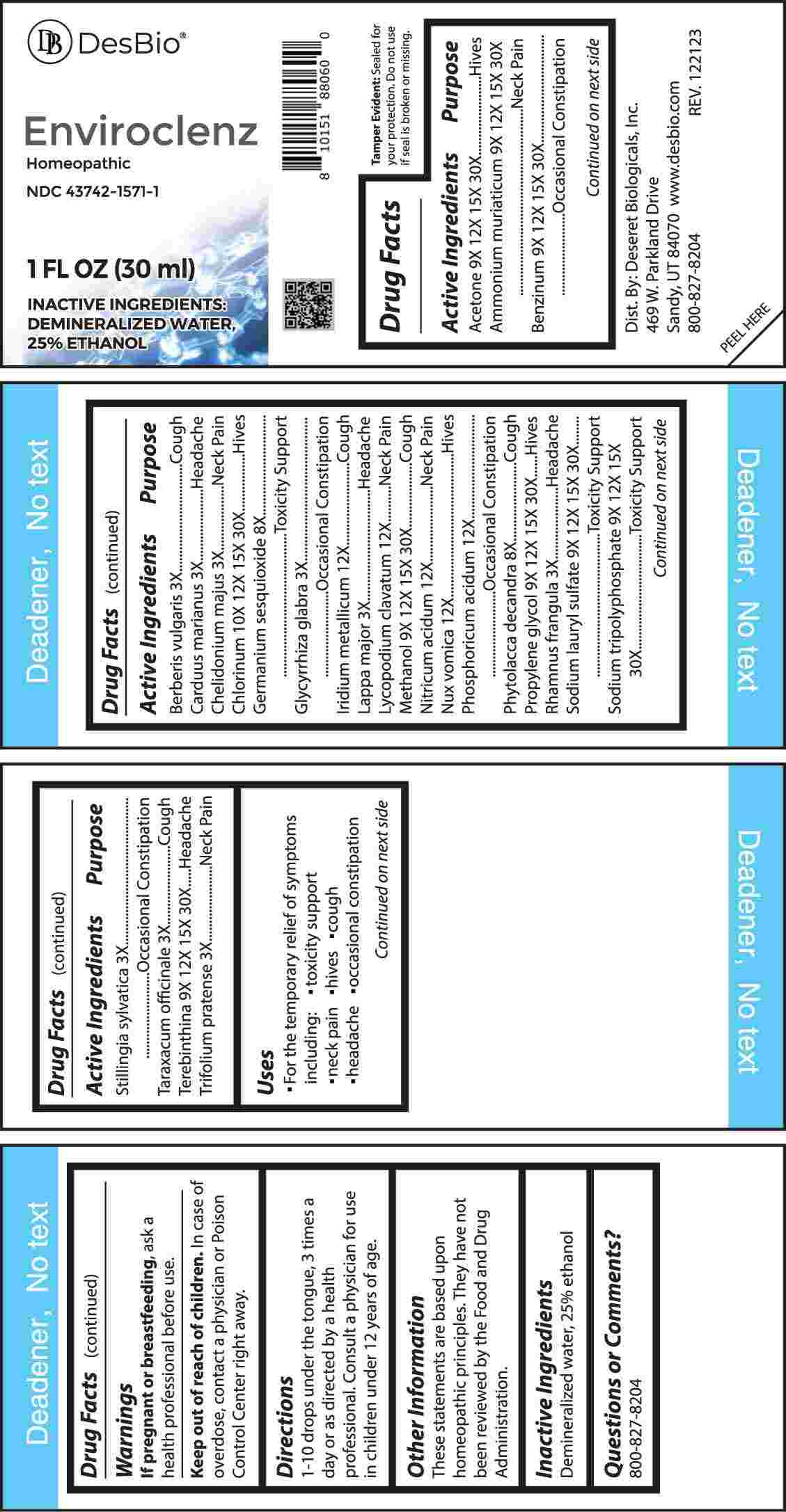
Label: ENVIROCLENZ- berberis vulgaris, carduus marianus, chelidonium majus, glycyrrhiza glabra, lappa major, rhamnus frangula, stillingia sylvatica, taraxacum officinale, trifolium pratense, germanium sesquioxide, phytolacca decandra, acetone, ammonium muriaticum, benzinum, propylene glycol, sodium lauryl sulfate, sodium tripolyphosphate, terebinthina, methanol, chlorinum, iridium metallicum, lycopodium clavatum, nitricum acidum, nux vomica, phosphoricum acidum liquid
- NDC Code(s): 43742-1571-1
- Packager: Deseret Biologicals, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated May 2, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENTS:
Acetone 9X, 12X, 15X, 30X, Ammonium Muriaticum 9X, 12X, 15X, 30X, Benzinum 9X, 12X, 15, 30X, Berberis Vulgaris 3X, Carduus Marianus 3X, Chelidonium Majus 3X, Chlorinum 10X, 12X, 15X, 30X, Germanium Sesquioxide 8X, Glycyrrhiza Glabra 3X, Iridium Metallicum 12X, Lappa Major 3X, Lycopodium Clavatum 12X, Methanol 9X, 12X, 15X, 30X, Nitricum Acidum 12X, Nux Vomica 12X, Phosphoricum Acidum 12X, Phytolacca Decandra 8X, , Propylene Glycol 9X, 12X, 15X, 30X, Rhamnus Frangula 3X, Sodium Lauryl Sulfate 9X, 12X, 15X, 30X, Sodium Tripolyphosphate 9X, 12X, 15X, Stillingia Sylvatica 3X, Taraxacum Officinale 3X, Terebinthina 9X, 12X, 15X, 30X, Trifolium Pratense 3X, 30X.
-
PURPOSE:
Acetone - Hives, Ammonium Muriaticum – Neck Pain, Benzinum – Occasional Constipation, Berberis Vulgaris - Cough, Carduus Marianus - Headache, Chelidonium Majus – Neck Pain, Chlorinum - Hives, Germanium Sesquioxide – Toxicity Support, Glycyrrhiza Glabra – Occasional Constipation, Iridium Metallicum - Cough, Lappa Major - Headache, Lycopodium Clavatum – Neck Pain, Methanol - Cough, Nitricum Acidum – Neck Pain, Nux Vomica - Hives, Phosphoricum Acidum – Occasional Constipation, Phytolacca Decandra - Cough, Propylene Glycol - Hives, Rhamnus Frangula - Headache, Sodium Lauryl Sulfate – Toxicity Support, Sodium Tripolyphosphate – Toxicity Support, Stillingia Sylvatica – Occasional Constipation, Taraxacum Officinale - Cough, Terebinthina - Headache, Trifolium Pratense – Neck Pain
- USES:
- WARNINGS:
- KEEP OUT OF REACH OF CHILDREN:
- DIRECTIONS:
- INACTIVE INGREDIENTS:
- QUESTIONS:
- PACKAGE LABEL DISPLAY:
-
INGREDIENTS AND APPEARANCE
ENVIROCLENZ
berberis vulgaris, carduus marianus, chelidonium majus, glycyrrhiza glabra, lappa major, rhamnus frangula, stillingia sylvatica, taraxacum officinale, trifolium pratense, germanium sesquioxide, phytolacca decandra, acetone, ammonium muriaticum, benzinum, propylene glycol, sodium lauryl sulfate, sodium tripolyphosphate, terebinthina, methanol, chlorinum, iridium metallicum, lycopodium clavatum, nitricum acidum, nux vomica, phosphoricum acidum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43742-1571 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BERBERIS VULGARIS ROOT BARK (UNII: 1TH8Q20J0U) (BERBERIS VULGARIS ROOT BARK - UNII:1TH8Q20J0U) BERBERIS VULGARIS ROOT BARK 3 [hp_X] in 1 mL MILK THISTLE (UNII: U946SH95EE) (MILK THISTLE - UNII:U946SH95EE) MILK THISTLE 3 [hp_X] in 1 mL CHELIDONIUM MAJUS WHOLE (UNII: 7E889U5RNN) (CHELIDONIUM MAJUS - UNII:7E889U5RNN) CHELIDONIUM MAJUS WHOLE 3 [hp_X] in 1 mL GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) (GLYCYRRHIZA GLABRA - UNII:2788Z9758H) GLYCYRRHIZA GLABRA 3 [hp_X] in 1 mL ARCTIUM LAPPA ROOT (UNII: 597E9BI3Z3) (ARCTIUM LAPPA ROOT - UNII:597E9BI3Z3) ARCTIUM LAPPA ROOT 3 [hp_X] in 1 mL FRANGULA ALNUS BARK (UNII: S2D77IH61R) (FRANGULA ALNUS BARK - UNII:S2D77IH61R) FRANGULA ALNUS BARK 3 [hp_X] in 1 mL STILLINGIA SYLVATICA ROOT (UNII: QBR70R4FBK) (STILLINGIA SYLVATICA ROOT - UNII:QBR70R4FBK) STILLINGIA SYLVATICA ROOT 3 [hp_X] in 1 mL TARAXACUM OFFICINALE FLOWERING TOP (UNII: DQS85W46HV) (TARAXACUM OFFICINALE FLOWERING TOP - UNII:DQS85W46HV) TARAXACUM OFFICINALE FLOWERING TOP 3 [hp_X] in 1 mL TRIFOLIUM PRATENSE LEAF (UNII: 612YOZ36DG) (TRIFOLIUM PRATENSE LEAF - UNII:612YOZ36DG) TRIFOLIUM PRATENSE LEAF 3 [hp_X] in 1 mL GERMANIUM SESQUIOXIDE (UNII: 96WE91N25T) (GERMANIUM SESQUIOXIDE - UNII:96WE91N25T) GERMANIUM SESQUIOXIDE 8 [hp_X] in 1 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 8 [hp_X] in 1 mL ACETONE (UNII: 1364PS73AF) (ACETONE - UNII:1364PS73AF) ACETONE 9 [hp_X] in 1 mL AMMONIUM CHLORIDE (UNII: 01Q9PC255D) (AMMONIUM CATION - UNII:54S68520I4) AMMONIUM CATION 9 [hp_X] in 1 mL BENZENE (UNII: J64922108F) (BENZENE - UNII:J64922108F) BENZENE 9 [hp_X] in 1 mL PROPYLENE GLYCOL (UNII: 6DC9Q167V3) (PROPYLENE GLYCOL - UNII:6DC9Q167V3) PROPYLENE GLYCOL 9 [hp_X] in 1 mL SODIUM LAURYL SULFATE (UNII: 368GB5141J) (LAURYL SULFATE - UNII:DIQ16UC154) SODIUM LAURYL SULFATE 9 [hp_X] in 1 mL SODIUM TRIPOLYPHOSPHATE (UNII: 5HK03SA80J) (TRIPOLYPHOSPHATE ION - UNII:5798IYA5AY) SODIUM TRIPOLYPHOSPHATE 9 [hp_X] in 1 mL TURPENTINE OIL (UNII: C5H0QJ6V7F) (TURPENTINE OIL - UNII:C5H0QJ6V7F) TURPENTINE OIL 9 [hp_X] in 1 mL METHYL ALCOHOL (UNII: Y4S76JWI15) (METHYL ALCOHOL - UNII:Y4S76JWI15) METHYL ALCOHOL 9 [hp_X] in 1 mL CHLORINE (UNII: 4R7X1O2820) (CHLORINE - UNII:4R7X1O2820) CHLORINE 10 [hp_X] in 1 mL IRIDIUM (UNII: 44448S9773) (IRIDIUM - UNII:44448S9773) IRIDIUM 12 [hp_X] in 1 mL LYCOPODIUM CLAVATUM SPORE (UNII: C88X29Y479) (LYCOPODIUM CLAVATUM SPORE - UNII:C88X29Y479) LYCOPODIUM CLAVATUM SPORE 12 [hp_X] in 1 mL NITRIC ACID (UNII: 411VRN1TV4) (NITRIC ACID - UNII:411VRN1TV4) NITRIC ACID 12 [hp_X] in 1 mL STRYCHNOS NUX-VOMICA SEED (UNII: 269XH13919) (STRYCHNOS NUX-VOMICA SEED - UNII:269XH13919) STRYCHNOS NUX-VOMICA SEED 12 [hp_X] in 1 mL PHOSPHORIC ACID (UNII: E4GA8884NN) (PHOSPHORIC ACID - UNII:E4GA8884NN) PHOSPHORIC ACID 12 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43742-1571-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 09/27/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 09/27/2019 Labeler - Deseret Biologicals, Inc. (940741853) Registrant - Apotheca Company (844330915) Establishment Name Address ID/FEI Business Operations Apotheca Company 844330915 manufacture(43742-1571) , api manufacture(43742-1571) , label(43742-1571) , pack(43742-1571)