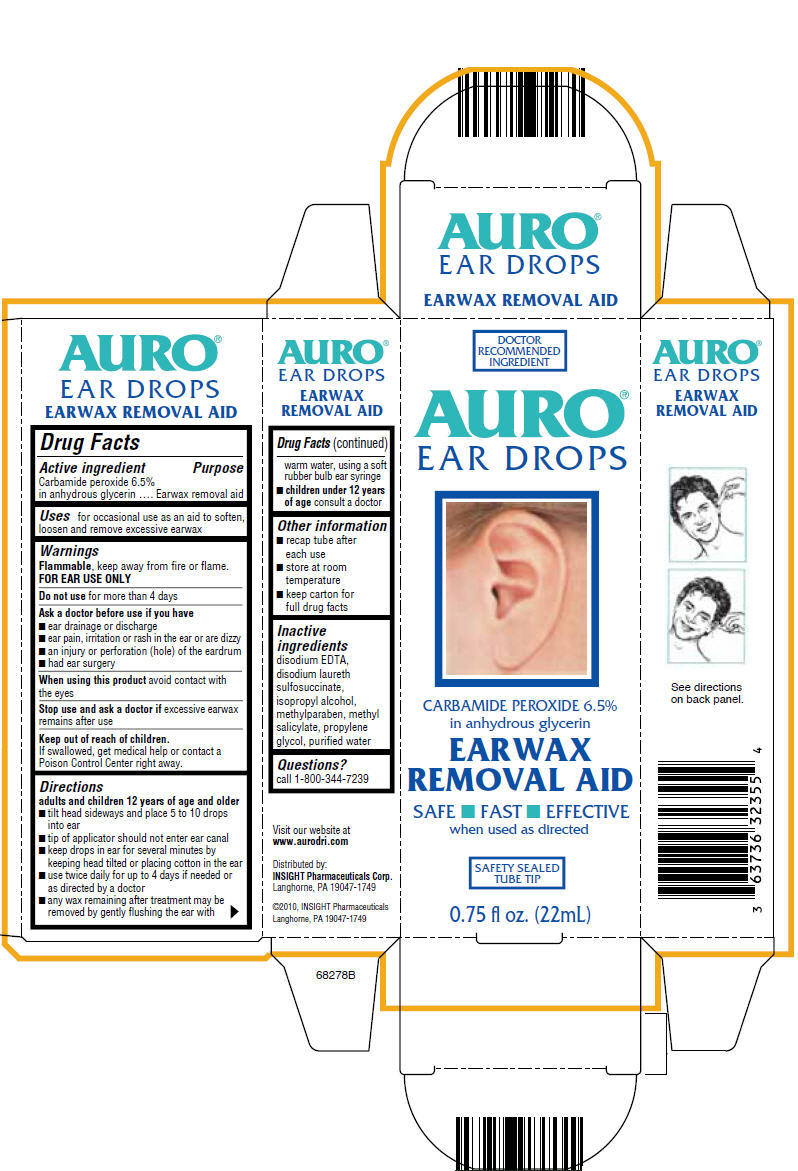
Label: AURO EAR WAX REMOVER- carbamide peroxide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 63736-232-24 - Packager: Insight Pharmaceuticals LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 31, 2011
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
-
Directions
adults and children 12 years of age and older
- tilt head sideways and place 5 to 10 drops into ear
- tip of applicator should not enter ear canal
- keep drops in ear for several minutes by keeping head tilted or placing cotton in the ear
- use twice daily for up to 4 days if needed or as directed by a doctor
- any wax remaining after treatment may be removed by gently flushing the ear with warm water, using a soft rubber bulb ear syringe
- children under 12 years of age consult a doctor
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 22mL Tube Carton
-
INGREDIENTS AND APPEARANCE
AURO EAR WAX REMOVER
carbamide peroxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63736-232 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Carbamide peroxide (UNII: 31PZ2VAU81) (Carbamide peroxide - UNII:31PZ2VAU81) Carbamide peroxide 1.44 mL in 22 mL Inactive Ingredients Ingredient Name Strength Edetate Disodium (UNII: 7FLD91C86K) Disodium Laureth Sulfosuccinate (UNII: D6DH1DTN7E) Isopropyl Alcohol (UNII: ND2M416302) Methylparaben (UNII: A2I8C7HI9T) Methyl Salicylate (UNII: LAV5U5022Y) Propylene Glycol (UNII: 6DC9Q167V3) Water (UNII: 059QF0KO0R) Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63736-232-24 1 in 1 BOX 1 22 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part344 07/01/2010 Labeler - Insight Pharmaceuticals LLC (176792315)