Label: SINUSIN- pulsatilla vulgaris and euphorbia resinifera resin and luffa opsilver nitrateerculata fruit and mercuric iodide and sus scrofa nasal mucosa and silver nitrate and calcium sulfide and sinusitisinum solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 51885-3150-1 - Packager: Biologische Heilmittel Heel
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated August 6, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- DOSAGE FORM
- CONTRAINDICATIONS
-
PRECAUTIONS
Pregnancy and nursing mothers: Pregnancy Category C. In general, homeopathic drugs are not known to cause direct or indirect harm to the fetus. However, animal reproduction studies have not been performed and there are no well-controlled studies in pregnant women. In cases of pregnancy or suspected pregnancy, a physician should be consulted before administering this drug. It is not known whether any of the ingredients are excreted in human milk. However because many drugs are excreted in human milk, homeopathic drugs should be administered with caution to nursing mothers.
Pediatric use: Homeopathic drugs can be safely administered to children. See Dosage and Administration.
- ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- WARNINGS
- HOW SUPPLIED
- STORAGE CONDITION
-
DOSAGE AND ADMINISTRATION
Adults and children above 6 years: In general 1 vial 1-3 times daily
Children to 6 years: 1/2 the adult dosage
Administration: NOT FOR INJECTION - FOR ORAL ADMINISTRATION ONLY.
Instructions for Opening Vial
1. Vials are pre-scored for easy opening and are marled with a color dot above the neck of the vial. The use of a file is not required. First, ensure that all contents of the vial are below the narrow neck by gently flicking or tapping top of the vial with fingers as in Step (1).
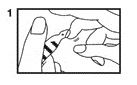
2. Hold vial firmly with one hand with dot toward you (2).
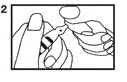
3. With the other hand, snap the vial top slightly upward and away from dot (3). Discard the vial top and be careful of sharp glass edges.
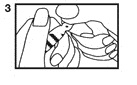
4. To withdraw solution: Tilt open vial until it is nearly horizontal (The vial should be tilted as far as possible without spilling the contents.) (4).
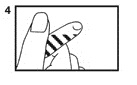
5. Squeeze bulb of the pipette and insert pipette tip into the solution (5).
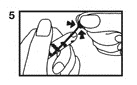
6. Slowly release pressure on the bulb of the pipette until solution is withdrawn from vial (6).

7. Remove pipette when the solution has been withdrawn (7).

Administer according to carton insert. Discard unused portion.
BE CAREFUL OF SHARP EDGES AFTER OPENING VIAL.
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SINUSIN
pulsatilla vulgaris and euphorbia resinifera resin and luffa opsilver nitrateerculata fruit and mercuric iodide and sus scrofa nasal mucosa and silver nitrate and calcium sulfide and sinusitisinum solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:51885-3150 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PULSATILLA VULGARIS (UNII: I76KB35JEV) (PULSATILLA VULGARIS - UNII:I76KB35JEV) PULSATILLA VULGARIS 2 [hp_X] in 2.2 mL EUPHORBIA RESINIFERA RESIN (UNII: 1TI1O9028K) (EUPHORBIA RESINIFERA RESIN - UNII:1TI1O9028K) EUPHORBIA RESINIFERA RESIN 4 [hp_X] in 2.2 mL LUFFA OPERCULATA FRUIT (UNII: C4MO6809HU) (LUFFA OPERCULATA FRUIT - UNII:C4MO6809HU) LUFFA OPERCULATA FRUIT 6 [hp_X] in 2.2 mL MERCURIC IODIDE (UNII: R03O05RB0P) (MERCURIC IODIDE - UNII:R03O05RB0P) MERCURIC IODIDE 8 [hp_X] in 2.2 mL SUS SCROFA NASAL MUCOSA (UNII: ID3Z1X61WY) (SUS SCROFA NASAL MUCOSA - UNII:ID3Z1X61WY) SUS SCROFA NASAL MUCOSA 8 [hp_X] in 2.2 mL SILVER NITRATE (UNII: 95IT3W8JZE) (SILVER CATION - UNII:57N7B0K90A) SILVER NITRATE 10 [hp_X] in 2.2 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (SULFATE ION - UNII:7IS9N8KPMG) CALCIUM SULFIDE 10 [hp_X] in 2.2 mL SINUSITISINUM (UNII: B575563DM5) (SINUSITISINUM - UNII:B575563DM5) SINUSITISINUM 13 [hp_X] in 2.2 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:51885-3150-1 2.2 mL in 1 VIAL, GLASS Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2011 Labeler - Biologische Heilmittel Heel (315635359)


