Label: SHINEPH- chamaecyparis obtusa leaf liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 73305-0001-1 - Packager: CH Bio Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated September 6, 2019
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
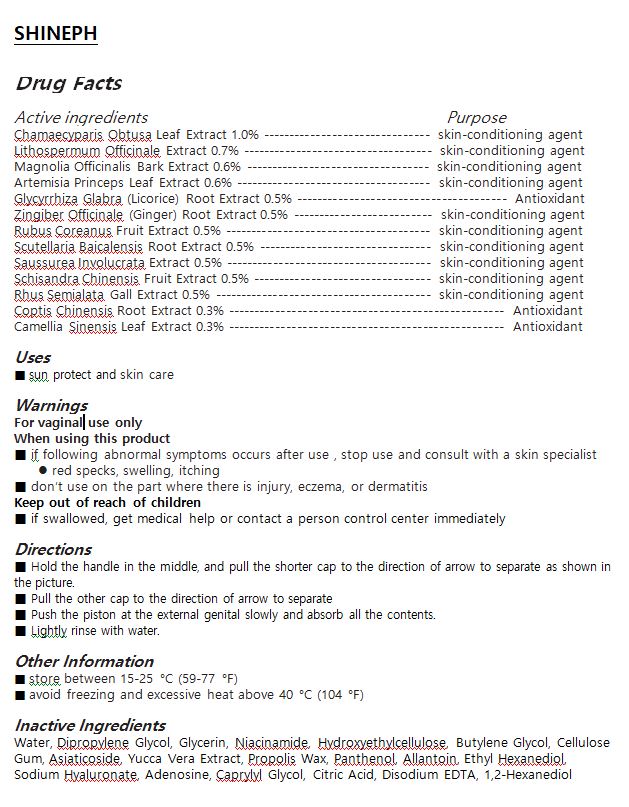
Chamaecyparis Obtusa Leaf extract, Lithospermum Officinale Extract, Magnolia Officinalis Bark Extract , Artemisia Princeps Leaf Extract , Glycyrrhiza Glabra (Licorice) Root Extract , Zingiber Officinale (Ginger) Root Extract , Rubus Coreanus Fruit Extract , Scutellaria Baicalensis Root Extract , Saussurea Involucrata Extract , Schisandra Chinensis Fruit Extract , Rhus Semialata Gall Extract , Coptis Chinensis Root Extract , Camellia Sinensis Leaf Extract
- INACTIVE INGREDIENT
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
-
WARNINGS
1 In the use of the product if the following phenomenon happen, please stop using; if continue using the situation gets worse, please consult with the doctor
1) red, itchy, tingling
2) please stop using if any of the above symptoms appear because of the sun exposure
2 Wounds, eczema, skin inflammation in the face, please do not use
3 Storage precautions
1) seale immediately after using
2) please place in theplace where the infant are unable to access in case eating by mistake
3) avoid placing in high temperature, low temperature or direct sunlight field - DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SHINEPH
chamaecyparis obtusa leaf liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73305-0001 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LITHOSPERMUM OFFICINALE ROOT (UNII: VOD5H80TXG) (LITHOSPERMUM OFFICINALE ROOT - UNII:VOD5H80TXG) LITHOSPERMUM OFFICINALE ROOT 0.7 g in 100 g ARTEMISIA PRINCEPS LEAF OIL (UNII: F9S1101A2V) (ARTEMISIA PRINCEPS LEAF OIL - UNII:F9S1101A2V) ARTEMISIA PRINCEPS LEAF OIL 0.6 g in 100 g MAGNOLIA OFFICINALIS BARK (UNII: 5M609NV974) (MAGNOLIA OFFICINALIS BARK - UNII:5M609NV974) MAGNOLIA OFFICINALIS BARK 0.6 g in 100 g CHAMAECYPARIS OBTUSA LEAF (UNII: 7OL154J5XB) (CHAMAECYPARIS OBTUSA LEAF - UNII:7OL154J5XB) CHAMAECYPARIS OBTUSA LEAF 1 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73305-0001-1 1.5 g in 1 APPLICATOR; Type 0: Not a Combination Product 09/06/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 08/17/2019 Labeler - CH Bio Co., Ltd. (694611809) Registrant - CH Bio Co., Ltd. (694611809) Establishment Name Address ID/FEI Business Operations CH Bio Co., Ltd. 694611809 manufacture(73305-0001) , label(73305-0001)