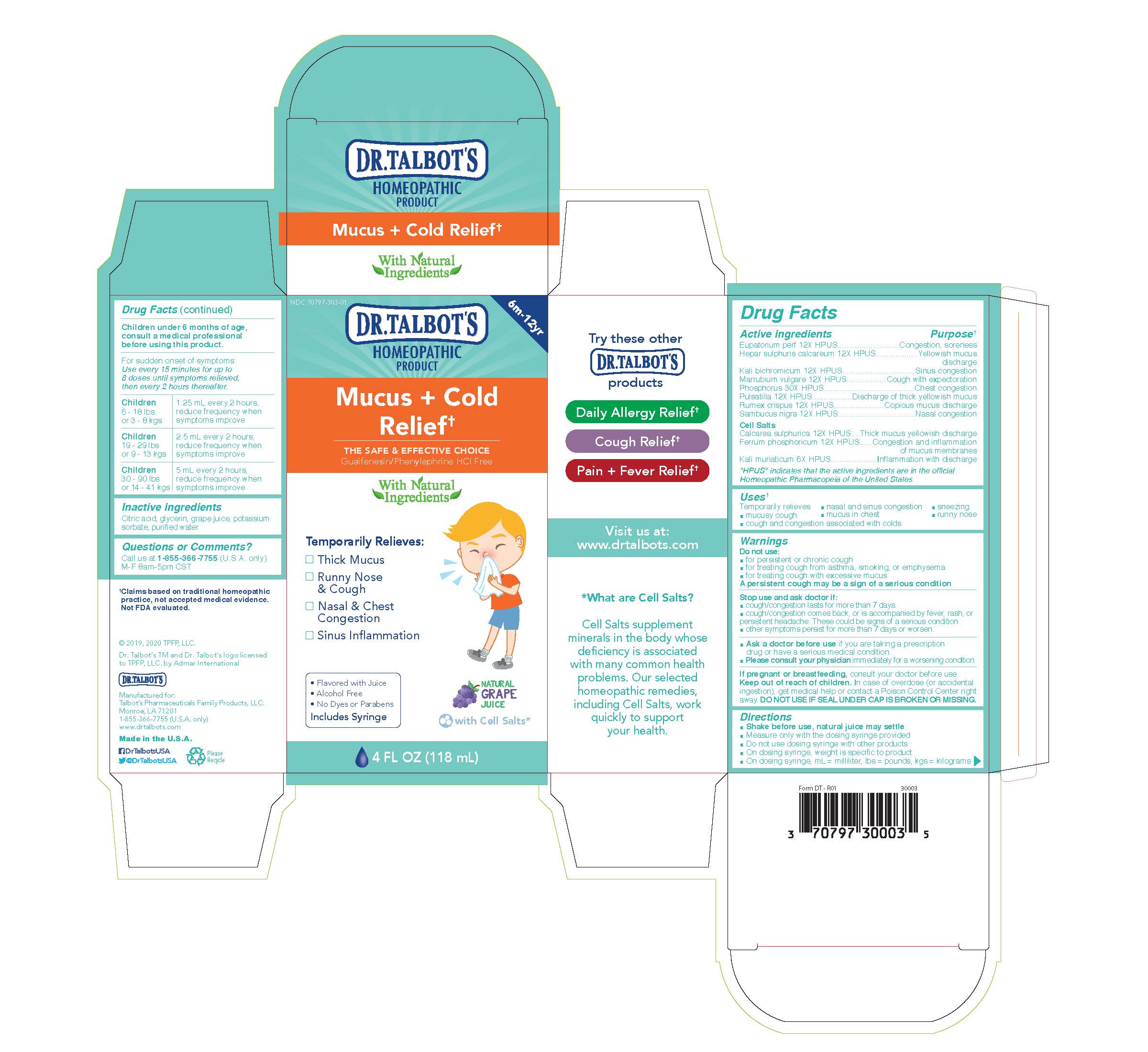
Label: DR. TALBOTS MUCUS COLD RELIEF- eupatorium perf, hepar sulphuris calcareum, kali bichromicum, marrubium vulgare, phosphorus, pulsatilla, rumex crispus, sambucus nigra, calcarea sulphurica, ferrum phosphoricum, kali muriaticum liquid
- NDC Code(s): 70797-313-01, 70797-313-04
- Packager: Talbot's Pharmaceuticals Family Products, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated October 5, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredients
Active Ingredients
Eupatorium perf 12X HPUS
Hepar sulphuris calcareum 12X HPUS
Kali bichromicum 12X HPUS
Marrubium vulgare 12X HPUS
Phosphorus 30X HPUS
Pulsatilla 12X HPUS
Rumex crispus 12X HPUS
Sambucus nigra 12x HPUS
Cell Salts
Calcarea sulphurica 12X HPUS
Ferrum phosphoricum 12X HPUS
Kali muriaticum 6X HPUS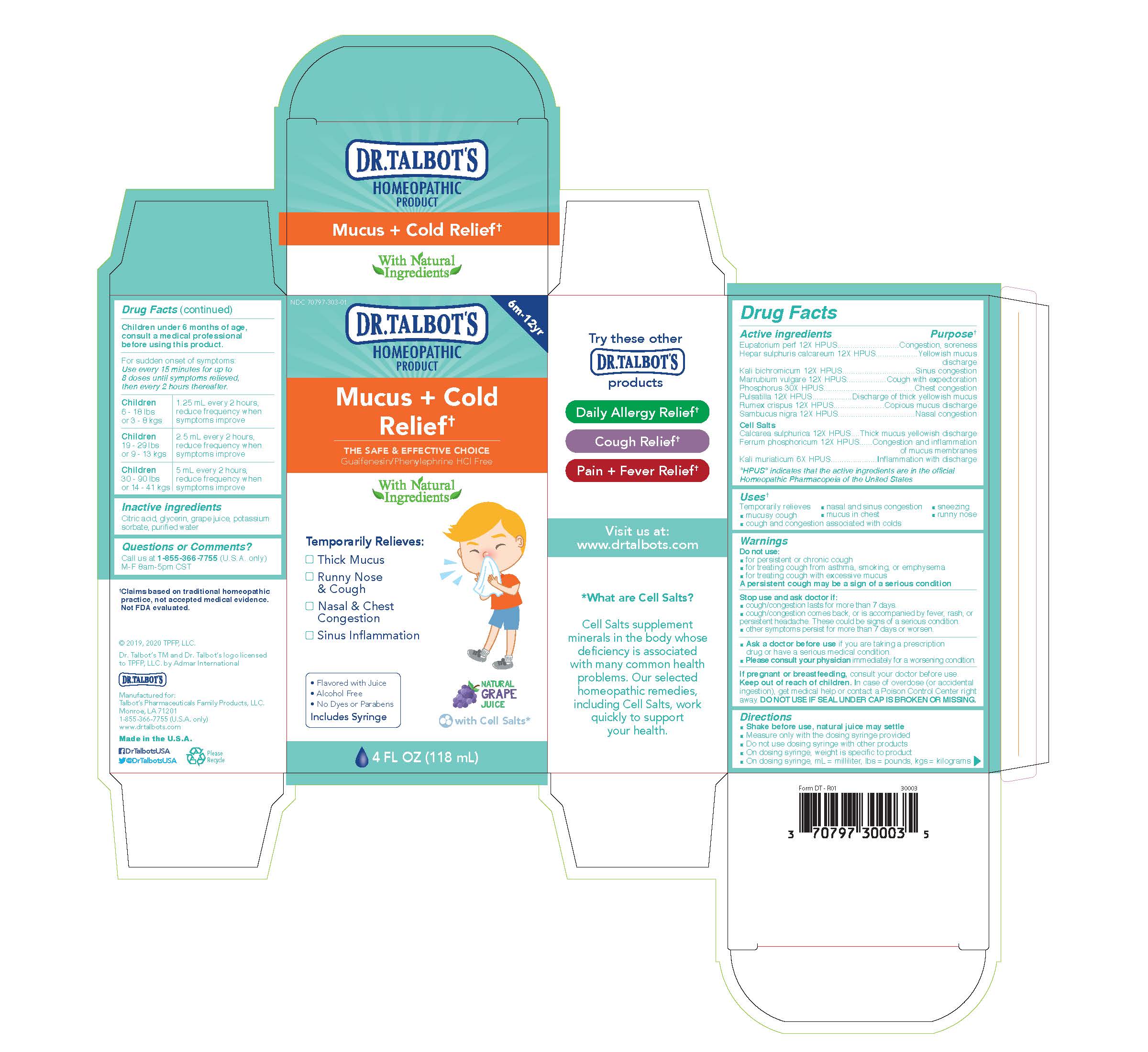
- Purpose
- Uses
-
Warnings
Do not use:
- for persistent or chronic cough
- for treating cough from asthma, smoking, or emphysema
- for treating cough with excessive mucus
A persistent cough may be a sign of a serious condition
Stop use and ask doctor if:
- cough/congestion lasts for more than 7 days.
- cough/congestion comes back, or is accompanied by fever, rash, or persistent headache. These could be signs of a serious confition.
- other symptoms persist for more than 7 days or worsen
- Ask a doctor before use if you are taking a prescription drug or have a serious medical condition.
- Please consult your physician immediately for a worsening condition.

- Pregnancy or breastfeeding
- Keep out of reach of children
-
Directions
- Shake before use, natural juice may settle
- Measure only with the dosing syringe provided
- Do not use dosing syringe with other products
- On dosing syringe, weight is specific to product
- On dosing syringe, ml = milliliter, lbs = pounds, kgs = kilograms
Children under 6 months of age, consult a medical professional before using this product.
For sudden onset of symptoms: Use every 15 minutes for up to 8 doses until symptoms relieved, then every 2 hours thereafter.
Children 6 - 18 lbs or 3 - 8 kgs
1.25 ml every 2 hours, reduce frequency when symptoms improve
Children 19 - 29 lbs or 9 - 13 kgs
2.5 ml every 2 hours, reduce frequency when symptoms improve
Children 30 - 90 lbs or 14 - 41 kgs
5 ml every 2 hours, reduce frequency when symptoms improve

- Inactive Ingredients
- Questions
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
DR. TALBOTS MUCUS COLD RELIEF
eupatorium perf, hepar sulphuris calcareum, kali bichromicum, marrubium vulgare, phosphorus, pulsatilla, rumex crispus, sambucus nigra, calcarea sulphurica, ferrum phosphoricum, kali muriaticum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70797-313 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EUPATORIUM PERFOLIATUM FLOWERING TOP (UNII: 1W0775VX6E) (EUPATORIUM PERFOLIATUM FLOWERING TOP - UNII:1W0775VX6E) EUPATORIUM PERFOLIATUM FLOWERING TOP 12 [hp_X] in 118 mL SAMBUCUS NIGRA FLOWERING TOP (UNII: CT03BSA18U) (SAMBUCUS NIGRA FLOWERING TOP - UNII:CT03BSA18U) SAMBUCUS NIGRA FLOWERING TOP 12 [hp_X] in 118 mL CALCIUM SULFATE ANHYDROUS (UNII: E934B3V59H) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE ANHYDROUS 12 [hp_X] in 118 mL POTASSIUM CHLORIDE (UNII: 660YQ98I10) (POTASSIUM CATION - UNII:295O53K152) POTASSIUM CHLORIDE 6 [hp_X] in 118 mL MARRUBIUM VULGARE (UNII: 7A72MUN24Z) (MARRUBIUM VULGARE - UNII:7A72MUN24Z) MARRUBIUM VULGARE 12 [hp_X] in 118 mL PHOSPHORUS (UNII: 27YLU75U4W) (PHOSPHORUS - UNII:27YLU75U4W) PHOSPHORUS 30 [hp_X] in 118 mL RUMEX CRISPUS ROOT (UNII: 9N1RM2S62C) (RUMEX CRISPUS ROOT - UNII:9N1RM2S62C) RUMEX CRISPUS ROOT 12 [hp_X] in 118 mL FERROSOFERRIC PHOSPHATE (UNII: 91GQH8I5F7) (FERROSOFERRIC PHOSPHATE - UNII:91GQH8I5F7) FERROSOFERRIC PHOSPHATE 12 [hp_X] in 118 mL CALCIUM SULFIDE (UNII: 1MBW07J51Q) (CALCIUM SULFIDE - UNII:1MBW07J51Q) CALCIUM SULFIDE 12 [hp_X] in 118 mL POTASSIUM DICHROMATE (UNII: T4423S18FM) (DICHROMATE ION - UNII:9LKY4BFN2V) POTASSIUM DICHROMATE 12 [hp_X] in 118 mL ANEMONE PULSATILLA (UNII: I76KB35JEV) (ANEMONE PULSATILLA - UNII:I76KB35JEV) ANEMONE PULSATILLA 12 [hp_X] in 118 mL Inactive Ingredients Ingredient Name Strength CONCORD GRAPE JUICE (UNII: F7039Q79LP) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70797-313-01 1 in 1 CARTON 07/20/2020 1 NDC:70797-313-04 118 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 07/20/2020 Labeler - Talbot's Pharmaceuticals Family Products, LLC. (078855555)