Label: RANOLAZINE tablet, film coated, extended release
- NDC Code(s): 71335-2556-1
- Packager: Bryant Ranch Prepack
- This is a repackaged label.
- Source NDC Code(s): 72319-021
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated January 23, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATIONHIGHLIGHTS OF PRESCRIBING INFORMATION - These highlights do not include all the information needed to use RANOLAZINE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing ...
-
Table of ContentsTable of Contents
-
1 INDICATIONS AND USAGE
Ranolazine Extended-Release Tablets are indicated for the treatment of chronic angina. Ranolazine Extended-Release Tablets may be used with beta-blockers, nitrates, calcium channel blockers ...
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing Information - Initiate Ranolazine Extended-Release Tablets dosing at 500 mg twice daily and increase to 1000 mg twice daily, as needed, based on clinical symptoms. Take Ranolazine ...
-
3 DOSAGE FORMS AND STRENGTHS
Ranolazine Extended-Release Tablets is supplied as film-coated, oval-shaped, extended-release tablets in the following strengths: • 500 mg tablets are orange, with I3 on one side and 21 on the ...
-
4 CONTRAINDICATIONS
Ranolazine Extended-Release Tablets is contraindicated in patients: • Taking strong inhibitors of CYP3A [see Drug Interactions ( 7.1)] • Taking inducers of CYP3A [see Drug Interactions ...
-
5 WARNINGS AND PRECAUTIONS
5.1 QT Interval Prolongation - Ranolazine blocks I - Kr and prolongs the QTc interval in a dose-related manner. Clinical experience in an acute coronary syndrome population did not show an ...
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience - Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly ...
-
7 DRUG INTERACTIONS
7.1 Effects of Other Drugs on Ranolazine - Strong CYP3A Inhibitors - Do not use Ranolazine Extended-Release Tablets with strong CYP3A inhibitors, including ketoconazole, itraconazole ...
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy - Risk Summary - There are no available data on Ranolazine Extended-Release Tablets use in pregnant women to inform any drug-associated risks. Studies in rats and rabbits showed ...
-
10 OVERDOSAGE
Hypotension, QT prolongation, bradycardia, myoclonic activity, severe tremor, unsteady - gait/incoordination, dizziness, nausea, vomiting, dysphasia, and hallucinations have been seen in cases of ...
-
11 DESCRIPTION
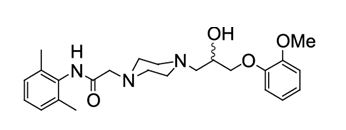
Ranolazine Extended-Release Tablets is available as a film-coated, non-scored, extended-release tablet for oral administration. Ranolazine is a racemic mixture, chemically described as ...
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action - The mechanism of action of ranolazine’s antianginal effects has not been determined. Ranolazine has anti-ischemic and antianginal effects that do not depend upon ...
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility - Ranolazine tested negative for genotoxic potential in the following assays: Ames bacterial mutation assay, Saccharomyces assay for ...
-
14 CLINICAL STUDIES
14.1 Chronic Stable Angina - CARISA (Combination Assessment of Ranolazine In Stable Angina) was a study in 823 chronic angina patients randomized to receive 12 weeks of treatment with ...
-
15 REFERENCES
M.A. Suckow et al. The anti-ischemia agent ranolazine promotes the development of intestinal tumors in APC (min/+) mice. Cancer Letters 209(2004):165−9.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Ranolazine Extended-Release Tablets is supplied as film-coated, oval-shaped, extended-release tablets: 500 mg tablets are orange, with I3 on one side and 21 on the other side. NDC: 71335-2556-1 ...
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information). Inform patients that Ranolazine Extended-Release Tablets will not abate an acute angina episode. Strong CY3PA ...
-
Patient Information
RANOLAZINE EXTENDED-RELEASE TABLETS - (ra-NOE-la-zeen) Dosing Strengths: 500 mg tablets - 1000 mg tablets - Read this Patient Information before you start taking Ranolazine ...
-
PRINCIPAL DISPLAY PANELRanolazine 500 mg ER Tablets
-
INGREDIENTS AND APPEARANCEProduct Information